324880 Sigma-Aldrich(–)-Epigallocatechin Gallate - CAS 989-51-5 - Calbiochem
One of the main polyphenolic constituents of green tea that exhibits potent antitumor, anti-inflammatory, and antioxidant properties.
More>> One of the main polyphenolic constituents of green tea that exhibits potent antitumor, anti-inflammatory, and antioxidant properties. Less<<Sinónimos: EGCG, (2R,3R)-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1[2H]-benzopyran-3,5,7-triol-3-(3,4,5-trihydroxybenzoate), HAT Inhibitor X, Histone Acetyltransferase Inhibitor X, p300/CBP Inhibitor VIII, PCAF Inhibitor V, DNA MTase Inhibitor IV, DNA Methyltransferase Inhibitor IV
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
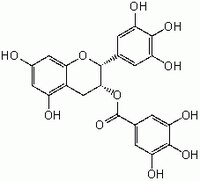
| 989-51-5 | C₂₂H₁₈O₁₁ |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 324880-10MG |
|
Ampolla de plást. | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 989-51-5 |
| ATP Competitive | N |
| Form | Faint pink solid |
| Hill Formula | C₂₂H₁₈O₁₁ |
| Chemical formula | C₂₂H₁₈O₁₁ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PMA-induced skin thickening |
| Primary Target IC<sub>50</sub> | 210-470 nM against Dnmt1; 30, 50, 60 and 70 µM for p300, CBP, PCAF and TIP60 |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | KB5200000 |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 324880-10MG | 04055977196092 |
Documentation
Licencias necesarias
| Título |
|---|
| CARTA DE USO GENERAL 2016 |
| PRODUCTO REGULADO POR LA SECRETARÍA DE SALUD |
(–)-Epigallocatechin Gallate - CAS 989-51-5 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
(–)-Epigallocatechin Gallate - CAS 989-51-5 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 324880 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Choi, K.C., et al. 2009. Cancer Res. 69, 583. Dell'Aicia, I., et al. 2004. EMBO reports 5, 1. Tachibana, H., et al. 2004. Nat. Struct. Mol. Biol. 11, 380. Naasami, I., et al. 1998. Biochem. Biophys. Res. Commun. 249, 391. Ahmad, N., et al. 1997. J. Natl. Cancer 89, 1881. Chan, M.M.-Y., et al. 1997. Biochem. Pharmacol. 54, 1281. Lin, Y.-L., and Lin, J.-K. 1997. Mol. Pharmacol. 52, 465. Fiala, E.S., et al. 1996. Experientia 52, 922. Katiyar, S.K., et al. 1995. J. Invest. Dermatol. 105, 394. Liao, S., et al. 1995. Cancer Lett. 96, 239. Yamane, T., et al. 1995. Cancer Res. 55, 2081. Huang, M.T., et al. 1992. Carcinogenesis 13, 947. |
Folleto
| Cargo |
|---|
| Caspases and other Apoptosis Related Tools Brochure |