506172 Sigma-Aldrichp38 MAP Kinase Inhibitor X, BIRB 796 - CAS 285983-48-4 - Calbiochem
p38 MAP Kinase Inhibitor X, BIRB 796, CAS 285983-48-4, is a cell-permeable, highly potent, slow binding, high affinity inhibitor of p38a (IC50 = 8 and 97 nM with or without 2 h preincubation).
More>> p38 MAP Kinase Inhibitor X, BIRB 796, CAS 285983-48-4, is a cell-permeable, highly potent, slow binding, high affinity inhibitor of p38a (IC50 = 8 and 97 nM with or without 2 h preincubation). Less<<Synonymes: 1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea, JNK Inhibitor XVII, Doramapimod, BIRB796
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 285983-48-4 | C₃₁H₃₇N₅O₃ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 506172-10MG |
|
Flacon en verre | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 285983-48-4 |
| Form | White solid |
| Hill Formula | C₃₁H₃₇N₅O₃ |
| Chemical formula | C₃₁H₃₇N₅O₃ |
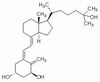
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 506172-10MG | 04055977272383 |
Documentation
p38 MAP Kinase Inhibitor X, BIRB 796 - CAS 285983-48-4 - Calbiochem FDS
| Titre |
|---|
p38 MAP Kinase Inhibitor X, BIRB 796 - CAS 285983-48-4 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 506172 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Liu. Y. and Gray, N.S., 2006. Nat. Chem. Biol. 2, 358. Regan, J., et al. 2003. J. Med. Chem. 46, 4676. Pargellis, C., et al. 2002. Nat. Struct. Biol. 9, 268. Regan, J., et al. 2002. J. Med. Chem. 45, 2994. |