480415 Sigma-AldrichNF023 - Calbiochem
A suramin analog that acts as a selective and direct G-protein antagonist for α-subunits of the Go/Gi group (EC₅₀ ~ 300 nM).
More>> A suramin analog that acts as a selective and direct G-protein antagonist for α-subunits of the Go/Gi group (EC₅₀ ~ 300 nM). Less<<Synonymes: 8,8ʹ-[Carbonylbis(imino-3,1-phenylene)]bis-(1,3,5-naphthalenetrisulfonic Acid), 6Na, P2X Antagonist I, Purinergic Receptor P2X Antagonist I
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| Empirical Formula |
|---|
| C₃₅H₂₀N₄O₂₁S₆ · 6Na |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 480415-10MG |
|
Ampoule plast. | 10 mg |
|
— |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | White to off-white solid |
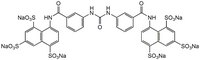
| Hill Formula | C₃₅H₂₀N₄O₂₁S₆ · 6Na |
| Chemical formula | C₃₅H₂₀N₄O₂₁S₆ · 6Na |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 480415-10MG | 04055977201345 |
Documentation
NF023 - Calbiochem FDS
| Titre |
|---|
NF023 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 480415 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Snedden, P., et al. 2000. Br. J. Pharmacol. 129, 1089. Soto, F., et al. 1999. Neuropharmacology 38, 141. Beindle, W., et al. 1996. Mol. Pharmacol. 50, 415. Freissmuth, M., et al. 1996. Mol. Pharmacol. 49, 602. |