382113 Sigma-AldrichHistone Acetyltransferase p300 Inhibitor, C646 - Calbiochem
Histone Acetyltransferase p300 Inhibitor, C646, CAS 328968-36-1, is a cell-permeable, reversible inhibitor of p300/CBP HAT (Ki = 400 nM). Competes with acetyl-CoA for the p300 Lys-CoA binding pocket.
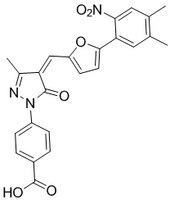
More>> Histone Acetyltransferase p300 Inhibitor, C646, CAS 328968-36-1, is a cell-permeable, reversible inhibitor of p300/CBP HAT (Ki = 400 nM). Competes with acetyl-CoA for the p300 Lys-CoA binding pocket. Less<<Synonymes: 4-(4-{[5-(4,5-dimethyl-2-nitrophenyl)furan-2-yl]methylidene}-3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)benzoic acid, p300/CBP Inhibitor IV, Histone Acetyltransferase Inhibitor V, HAT Inhibitor V
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 328968-36-1 | C₂₄H₁₉N₃O₆ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 382113-10MG |
|
Ampoule plast. | 10 mg |
|
— |
| References | |
|---|---|
| References | Bowers, EM., et al. 2010. Chem Biol 17, 471. |
| Product Information | |
|---|---|
| CAS number | 328968-36-1 |
| Form | Brick red solid |
| Hill Formula | C₂₄H₁₉N₃O₆ |
| Chemical formula | C₂₄H₁₉N₃O₆ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥99% by HPLC (sum of isomers) |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 382113-10MG | 04055977213119 |
Documentation
Histone Acetyltransferase p300 Inhibitor, C646 - Calbiochem FDS
| Titre |
|---|
Histone Acetyltransferase p300 Inhibitor, C646 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 382113 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Bowers, EM., et al. 2010. Chem Biol 17, 471. |
Informations techniques
| Titre |
|---|
| White Paper - The Message in the Marks: Deciphering Cancer Epigenetics |