196322 Sigma-AldrichBaicalein - CAS 491-67-8 - Calbiochem
A cell-permeable flavone that inhibits the activity of 12-lipoxygenase (IC₅₀= 120 nM) and reverse transcriptase.
More>> A cell-permeable flavone that inhibits the activity of 12-lipoxygenase (IC₅₀= 120 nM) and reverse transcriptase. Less<<Synonymes: 5,6,7-Trihydroxyflavone
Produits recommandés
Aperçu
| Replacement Information |
|---|
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 196322-10MG |
|
Ampoule plast. | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 491-67-8 |
| ATP Competitive | N |
| Form | Yellow solid |
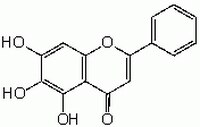
| Hill Formula | C₁₅H₁₀O₅ |
| Chemical formula | C₁₅H₁₀O₅ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | 12-lipoxygenase |
| Primary Target IC<sub>50</sub> | 120 nM against 12-lipoxygenase |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 196322-10MG | 04055977206463 |
Documentation
Baicalein - CAS 491-67-8 - Calbiochem FDS
| Titre |
|---|
Baicalein - CAS 491-67-8 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 196322 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Leabeau, A., et al. 2001. Neuroreport 12, 2199. Gao, D., et al. 1996. Biochem. Mol. Biol. Int. 39, 215. Matsuzaki, Y., et al. 1996. Jpn. J. Cancer Res. 87, 170. Huang, H.C., et al. 1994. Eur. J. Pharmacol. 268, 73. Butenko, I.G., et al. 1993. Agents Actions 39, C49. Abe, K., et al. 1990. Chem. Pharm. Bull. 38, 209. Kimura, Y., et al. 1987. Biochim. Biophys. Acta 922, 278. Sekiya, K., and Okuda, H. 1982. Biochem. Biophys. Res. Commun. 105, 1090. |