196876 Sigma-AldrichBAY 41-2272 - CAS 256376-24-6 - Calbiochem
A cell-permeable pyrazolopyridinylpyrimidine compound that acts as a selective and potent stimulator of soluble guanylate cyclase (effective dose ~ 0.1 nM to 100 µM using recombinant soluble guanylate cyclase).
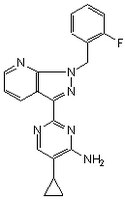
More>> A cell-permeable pyrazolopyridinylpyrimidine compound that acts as a selective and potent stimulator of soluble guanylate cyclase (effective dose ~ 0.1 nM to 100 µM using recombinant soluble guanylate cyclase). Less<<Synonymes: 5-Cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-4-ylamine
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 256376-24-6 | C₂₀H₁₇FN₆ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 196876-5MG |
|
Ampoule plast. | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 256376-24-6 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₂₀H₁₇FN₆ |
| Chemical formula | C₂₀H₁₇FN₆ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 196876-5MG | 04055977206593 |
Documentation
BAY 41-2272 - CAS 256376-24-6 - Calbiochem FDS
| Titre |
|---|
BAY 41-2272 - CAS 256376-24-6 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 196876 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Boerrigter, G., et al. 2003. Circulation 107, 686. Kalsi, J.S., et al. 2003. J Urol. 169, 761. Koglin, M., et al. 2002. Biochem. Biophys. Res. Commun. 292, 1057. Becker, E.M., et al. 2001. BMC Pharmacol. 1, 13. Stasch, J.P., et al. 2001. Nature 410, 212. |