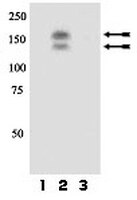
c-Met modulates RPE migratory response to laser-induced retinal injury.
Kasaoka, M; Ma, J; Lashkari, K
PloS one
7
e40771
2011
Afficher le résumé
Retinal laser injuries are often associated with aberrant migration of the retinal pigment epithelium (RPE), which can cause expansion of the scar beyond the confines of the original laser burn. In this study, we devised a novel method of laser-induced injury to the RPE layer in mouse models and began to dissect the mechanisms associated with pathogenesis and progression of laser-induced RPE injury. We have hypothesized that the proto-oncogene receptor, c-Met, is intimately involved with migration of RPE cells, and may be an early responder to injury. Using transgenic mouse models, we show that constitutive activation of c-Met induces more robust RPE migration into the outer retina of laser-injured eyes, while abrogation of the receptor using a cre-lox method reduces these responses. We also demonstrate that retinal laser injury increases expression of both HGF and c-Met, and activation of c-Met after injury is correlated with RPE cell migration. RPE migration may be responsible for clinically significant anatomic changes observed after laser injury. Abrogation of c-Met activity may be a therapeutic target to minimize retinal damage from aberrant RPE cell migration. | Immunohistochemistry | 22808260
 |
Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1.
Palka, Helena L, et al.
J. Biol. Chem., 278: 5728-35 (2003)
2003
Afficher le résumé
The receptor protein-tyrosine phosphatase (PTP) DEP-1 (CD148/PTP-eta) has been implicated in the regulation of cell growth, differentiation, and transformation, and most recently has been identified as a potential tumor suppressor gene mutated in colon, lung, and breast cancers. We have generated constructs comprising the cytoplasmic segment of DEP-1 fused to the maltose-binding protein to identify potential substrates and thereby suggest a physiological function for DEP-1. We have shown that the substrate-trapping mutant form of DEP-1 interacted with a small subset of tyrosine-phosphorylated proteins from lysates of the human breast tumor cell lines MDA-MB-231, T-47D, and T-47D/Met and have identified the hepatocyte growth factor/scatter factor receptor Met, the adapter protein Gab1, and the junctional component p120 catenin as potential substrates. Following ligand stimulation, phosphorylation of specific tyrosyl residues in Met induces mitogenic, motogenic, and morphogenic responses. When co-expressed in 293 cells, the full-length substrate-trapping mutant form of DEP-1 formed a stable complex with the chimeric receptor colony stimulating factor 1 (CSF)-Met and wild type DEP-1 dephosphorylated CSF-Met. Furthermore, we observed that DEP-1 preferentially dephosphorylated a Gab1 binding site (Tyr(1349)) and a COOH-terminal tyrosine implicated in morphogenesis (Tyr(1365)), whereas tyrosine residues in the activation loop of Met (Tyr(1230), Tyr(1234), and Tyr(1235)) were not preferred targets of the PTP. The ability of DEP-1 preferentially to dephosphorylate particular tyrosine residues that are required for Met-induced signaling suggests that DEP-1 may function in controlling the specificity of signals induced by this PTK, rather than as a simple "off-switch" to counteract PTK activity. | | 12475979
 |
Tyrosines1234-1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor).
Longati, P, et al.
Oncogene, 9: 49-57 (1994)
1993
Afficher le résumé
The tyrosine kinase encoded by the MET proto-oncogene (p190MET) is the receptor for Hepatocyte Growth Factor/Scatter Factor (HGF/SF). Previous work has shown that autophosphorylation of p190MET enhances its enzymatic activity and that the major phosphorylation site is Tyr1235, located in the catalytic domain. This residue is part of a 'three tyrosine' motif, including Tyr1230, Tyr1234, and Tyr1235, conserved in several other receptor kinases. We studied the role of these tyrosines in the positive regulation of the p190MET kinase by site-directed mutagenesis. Substitution of either Tyr1235 or Tyr1234 with phenylalanine severely reduced the in vitro kinase activity toward exogenous substrates. Kinetic experiments showed that the residual activity of these mutants could still be enhanced by autophosphorylation. Phosphopeptide mapping indicated that, in the absence of Tyr1235, Tyr1234 is phosphorylated. Only the replacement of both Tyr1234 and Tyr1235 yielded a mutant which completely lost the ability to be activated by autophosphorylation. In stable transfectants expressing the HGF/SF receptor with single substitution of either Tyr1234 or Tyr1235 the response to HGF/SF was impaired. The ligand did not induce tyrosine phosphorylation of the receptor nor stimulated chemotaxis. These data show that Tyr1234 and Tyr1235 are critical for the activation of the HGF/SF receptor kinase both in vitro and in response to the ligand in intact cells. | | 8302603
 |