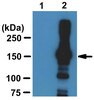
NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176.
Ling, L, et al.
Proc. Natl. Acad. Sci. U.S.A., 95: 3792-7 (1998)
1998
Afficher le résumé
Activation of the transcription factor NF-kappaB by inflammatory cytokines involves the successive action of NF-kappaB-inducing kinase (NIK) and two IkappaB kinases, IKK-alpha and IKK-beta. Here we show that NIK preferentially phosphorylates IKK-alpha over IKK-beta, leading to the activation of IKK-alpha kinase activity. This phosphorylation of IKK-alpha occurs specifically on Ser-176 in the activation loop between kinase subdomains VII and VIII. A mutant form of IKK-alpha containing alanine at residue 176 cannot be phosphorylated or activated by NIK and acts as a dominant negative inhibitor of interleukin 1- and tumor necrosis factor-induced NF-kappaB activation. Conversely, a mutant form of IKK-alpha containing glutamic acid at residue 176 is constitutively active. Thus, the phosphorylation of IKK-alpha on Ser-176 by NIK may be required for cytokine-mediated NF-kappaB activation. | 9520446
 |
IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK.
Woronicz, J D, et al.
Science, 278: 866-9 (1997)
1997
Afficher le résumé
Activation of the transcription factor nuclear factor kappa B (NF-kappaB) by inflammatory cytokines requires the successive action of NF-kappaB-inducing kinase (NIK) and IkappaB kinase-alpha (IKK-alpha). A widely expressed protein kinase was identified that is 52 percent identical to IKK-alpha. IkappaB kinase-beta (IKK-beta) activated NF-kappaB when overexpressed and phosphorylated serine residues 32 and 36 of IkappaB-alpha and serines 19 and 23 of IkappaB-beta. The activity of IKK-beta was stimulated by tumor necrosis factor and interleukin-1 treatment. IKK-alpha and IKK-beta formed heterodimers that interacted with NIK. Overexpression of a catalytically inactive form of IKK-beta blocked cytokine-induced NF-kappaB activation. Thus, an active IkappaB kinase complex may require three distinct protein kinases. | 9346485
 |
MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1.
Malinin, N L, et al.
Nature, 385: 540-4 (1997)
1997
Afficher le résumé
Several members of the tumour-necrosis/nerve-growth factor (TNF/NGF) receptor family activate the transcription factor NF-kappaB through a common adaptor protein, Traf2 (refs 1-5), whereas the interleukin 1 type-I receptor activates NF-kappaB independently of Traf2 (ref. 4). We have now cloned a new protein kinase, NIK, which binds to Traf2 and stimulates NF-kappaB activity. This kinase shares sequence similarity with several MAPKK kinases. Expression in cells of kinase-deficient NIK mutants fails to stimulate NF-kappaB and blocks its induction by TNF, by either of the two TNF receptors or by the receptor CD95 (Fas/Apo-1), and by TRADD, RIP and MORT1/FADD, which are adaptor proteins that bind to these receptors. It also blocked NF-kappaB induction by interleukin-1. Our findings indicate that NIK participates in an NF-kappaB-inducing signalling cascade common to receptors of the TNF/NGF family and to the interleukin-1 type-I receptor. | 9020361
 |