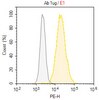
The proteolytic activity of MT4-MMP is required for its pro-angiogenic and pro-metastatic promoting effects.
Lorin Host,Alexandra Paye,Benoit Detry,Silvia Blacher,Carine Munaut,Jean Michel Foidart,Motoharu Seiki,Nor Eddine Sounni,Agnès Noel,Agn Noel
International journal of cancer. Journal international du cancer
131
2011
Afficher le résumé
Membrane-type 4 matrix metalloprotease (MT4-MMP) expression in breast adenocarcinoma stimulates tumor growth and metastatic spreading to the lung. However, whether these pro-tumorigenic and pro-metastatic effects of MT4-MMP are related to a proteolytic action is not yet known. Through site directed mutagenesis MT4-MMP has been inactivated in cancer cells through Glutamic acid 249 substitution by Alanine in the active site. Active MT4-MMP triggered an angiogenic switch at day 7 after tumor implantation and drastically accelerated subcutaneous tumor growth as well as lung colonization in recombination activating gene-1-deficient mice. All these effects were abrogated upon MT4-MMP inactivation. In sharp contrast to most MMPs being primarily of stromal origin, we provide evidence that tumor-derived MT4-MMP, but not host-derived MT4-MMP contributes to angiogenesis. A genetic approach using MT4-MMP-deficient mice revealed that the status of MT4-MMP produced by host cells did not affect the angiogenic response. Despite of this tumor intrinsic feature, to exert its tumor promoting effect, MT4-MMP requires a permissive microenvironment. Indeed, tumor-derived MT4-MMP failed to circumvent the lack of an host angio-promoting factor such as plasminogen activator inhibitor-1. Overall, our study demonstrates the key contribution of MT4-MMP catalytic activity in the tumor compartment, at the interface with host cells. It identifies MT4-MMP as a key intrinsic tumor cell determinant that contributes to the elaboration of a permissive microenvironment for metastatic dissemination. | 22262494
 |
Insights into ectodomain shedding and processing of protein-tyrosine pseudokinase 7 (PTK7).
Golubkov, VS; Strongin, AY
The Journal of biological chemistry
287
42009-18
2011
Afficher le résumé
The membrane PTK7 pseudokinase, a component of both the canonical and noncanonical/planar cell polarity Wnt pathways, modulates cell polarity and motility in biological processes as diverse as embryo development and cancer cell invasion. To determine the individual proteolytic events and biological significance of the ectodomain shedding in the PTK7 function, we used highly invasive fibrosarcoma HT1080 cells as a model system. Current evidence suggested a likely link between PTK7 shedding and cell invasion in our HT1080 cell model system. We also demonstrated that in HT1080 cells the cleavage of the PTK7 ectodomain by an ADAM proteinase was coupled with the membrane type-1 matrix metalloproteinase (MT1-MMP) cleavage of the PKP(621)↓LI site in the seventh Ig-like domain of PTK7. Proteolytic cleavages led to the generation of two soluble, N-terminal and two matching C-terminal, cell-associated fragments of PTK7. This proteolysis was a prerequisite for the intramembrane cleavage of the C-terminal fragments of PTK7 by γ-secretase. γ-Secretase cleavage was predominantly followed by the efficient decay of the resulting C-terminal PTK7 fragment via the proteasome. In contrast, in HT1080 cells, which overexpressed the C-terminal PTK7 fragment, the latter readily entered the nucleus. Our data imply that therapeutic inhibition of PTK7 shedding may be used to slow cancer progression. | 23095747
 |
Potential relation of aberrant proteolysis of human protein tyrosine kinase 7 (PTK7) chuzhoi by membrane type 1 matrix metalloproteinase (MT1-MMP) to congenital defects.
Vladislav S Golubkov,Alexander E Aleshin,Alex Y Strongin
The Journal of biological chemistry
286
2010
Afficher le résumé
Membrane PTK7 pseudo-kinase plays an essential role in planar cell polarity and the non-canonical Wnt pathway in vertebrates. Recently, a new N-ethyl-N-nitrosourea-induced mutant named chuzhoi (chz) was isolated in mice. chz embryos have severe birth defects, including a defective neural tube, defective heart and lung development, and a shortened anterior-posterior body axis. The chz mutation was mapped to the Ala-Asn-Pro tripeptide insertion into the junction region between the fifth and the sixth Ig-like domains of PTK7. Unexpectedly, chz reduced membrane localization of the PTK7 protein. We hypothesized and then proved that the chz mutation caused an insertion of an additional membrane type 1 matrix metalloproteinase cleavage site in PTK7 and that the resulting aberrant proteolysis of chz affected the migratory parameters of the cells. It is likely that aberrations in the membrane type 1 matrix metalloproteinase/PTK7 axis are detrimental to cell movements that shape the body plan and that chz represents a novel model system for increasing our understanding of the role of proteolysis in developmental pathologies, including congenital defects. | 21518755
 |
The Wnt/planar cell polarity protein-tyrosine kinase-7 (PTK7) is a highly efficient proteolytic target of membrane type-1 matrix metalloproteinase: implications in cancer and embryogenesis.
Golubkov, VS; Chekanov, AV; Cieplak, P; Aleshin, AE; Chernov, AV; Zhu, W; Radichev, IA; Zhang, D; Dong, PD; Strongin, AY
The Journal of biological chemistry
285
35740-9
2009
Afficher le résumé
PTK7 is an essential component of the Wnt/planar cell polarity (PCP) pathway. We provide evidence that the Wnt/PCP pathway converges with pericellular proteolysis in both normal development and cancer. Here, we demonstrate that membrane type-1 matrix metalloproteinase (MT1-MMP), a key proinvasive proteinase, functions as a principal sheddase of PTK7. MT1-MMP directly cleaves the exposed PKP(621)↓LI sequence of the seventh Ig-like domain of the full-length membrane PTK7 and generates, as a result, an N-terminal, soluble PTK7 fragment (sPTK7). The enforced expression of membrane PTK7 in cancer cells leads to the actin cytoskeleton reorganization and the inhibition of cell invasion. MT1-MMP silencing and the analysis of the uncleavable L622D PTK7 mutant confirm the significance of MT1-MMP proteolysis of PTK7 in cell functions. Our data also demonstrate that a fine balance between the metalloproteinase activity and PTK7 levels is required for normal development of zebrafish (Danio rerio). Aberration of this balance by the proteinase inhibition or PTK7 silencing results in the PCP-dependent convergent extension defects in the zebrafish. Overall, our data suggest that the MT1-MMP-PTK7 axis plays an important role in both cancer cell invasion and normal embryogenesis in vertebrates. Further insight into these novel mechanisms may promote understanding of directional cell motility and lead to the identification of therapeutics to treat PCP-related developmental disorders and malignancy. Article en texte intégral | 20837484
 |
Posttranslational regulation of membrane type 1-matrix metalloproteinase (MT1-MMP) in mouse PTEN null prostate cancer cells: Enhanced surface expression and differential O-glycosylation of MT1-MMP.
Kim, S; Huang, W; Mottillo, EP; Sohail, A; Ham, YA; Conley-Lacomb, MK; Kim, CJ; Tzivion, G; Kim, HR; Wang, S; Chen, YQ; Fridman, R
Biochimica et biophysica acta
1803
1287-97
2009
Afficher le résumé
Membrane type 1 (MT1)-matrix metalloproteinase (MT1-MMP) is a membrane-tethered MMP that has been shown to play a key role in promoting cancer cell invasion. MT1-MMP is highly expressed in bone metastasis of prostate cancer (PC) patients and promotes intraosseous tumor growth of PC cells in mice. The majority of metastatic prostate cancers harbor loss-of-function mutations or deletions of the tumor suppressor PTEN (phosphatase and tensin homologue deleted on chromosome ten). However, the role of PTEN inactivation in MT1-MMP expression in PC cells has not been examined. In this study, prostate epithelial cell lines derived from mice that are either heterozygous (PTEN(+/-)) or homozygous (PTEN(-/-)) for PTEN deletion or harboring a wild-type PTEN (PTEN(+/+)) were used to investigate the expression of MT1-MMP. We found that biallelic loss of PTEN is associated with posttranslational regulation of MT1-MMP protein in mouse PC cells. PTEN(-/-) PC cells display higher levels of MT1-MMP at the cell surface when compared to PTEN(+/+) and PTEN(+/-) cells and consequently exhibited enhanced migratory and collagen-invasive activities. MT1-MMP displayed by PTEN(-/-) cells is differentially O-glycosylated and exhibits a slow rate of turnover. MT1-MMP expression in PTEN(-/-) cells is under control of the PI3K/AKT signaling pathway, as determined using pharmacological inhibitors. Interestingly, rapamycin, an mTOR inhibitor, upregulates MT1-MMP expression in PTEN(+/+) cells via PI3K activity. Collectively, these data in a mouse prostate cell system uncover for the first time a novel and complex relationship between PTEN loss-mediated PI3K/AKT activation and posttranslational regulation of MT1-MMP, which may play a role in PC progression. Article en texte intégral | 20620173
 |
















aminobenzoph[814473_-(Benzylprolyl)aminobenzoph-ALL].jpg)