Combination treatment with ethyl pyruvate and IGF-I exerts neuroprotective effects against brain injury in a rat model of neonatal hypoxic-ischemic encephalopathy.
Rong, Z; Pan, R; Chang, L; Lee, W
International journal of molecular medicine
36
195-203
2015
Afficher le résumé
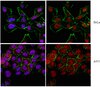
Neonatal hypoxic-ischemic (HI) brain injury causes severe brain damage in newborns. Following HI injury, rapidly accumulating oxidants injure neurons and interrupt ongoing developmental processes. The antioxidant, sodium pyruvate, has been shown to reduce neuronal injury in neonatal rats under conditions of oxygen glucose deprivation (OGD) and HI injury. In this study, we evaluated the effects of ethyl pyruvate (EP) and insulin‑like growth factor‑I (IGF‑I) alone or in combination in a similar setting. For this purpose, we used an in vitro model involving primary neonatal rat cortical neurons subjected to OGD for 2.5 h and an in vivo model involving unilateral carotid ligation in rats on post-natal day 7 with exposure to 8% hypoxia for 2.5 h. The cultured neurons were examined by lactate dehydrogenase (LDH) and cell viability assays. For the in vivo experiments, behavioral development was evaluated by the foot fault test at 4 weeks of recovery. 2,3,5‑Triphenyltetrazolium chloride monohydrate and cresyl violet staining were used to evaluate HI injury. The injured neurons were Fluoro‑Jade B-labeled, new neuroprecursors were double labeled with bromodeoxyuridine (BrdU) and doublecortin, new mature neurons were BrdU-labeled and neuronal nuclei were labeled by immunofluorescence. Under conditions of OGD, the LDH levels increased and neuronal viability decreased. Treatment with 0.5 mM EP or 25 ng/ml IGF‑I protected the neurons (Pless than 0.05), exerting additive effects. Similarly, either the early administration of EP or delayed treatment with IGF‑I protected the neonatal rat brains against HI injury and improved neurological performance and these effects were also additive. This effect may be the result of reduced neuronal injury, and enhanced neurogenesis and maturation. On the whole, our findings demonstrate that the combination of the early administration of EP with delayed treatment with IGF‑I exerts neuroprotective effects against HI injury in neonatal rat brains. | 25999282
 |
Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal.
Yousef, H; Conboy, MJ; Morgenthaler, A; Schlesinger, C; Bugaj, L; Paliwal, P; Greer, C; Conboy, IM; Schaffer, D
Oncotarget
6
11959-78
2015
Afficher le résumé
Stem cell function declines with age largely due to the biochemical imbalances in their tissue niches, and this work demonstrates that aging imposes an elevation in transforming growth factor β (TGF-β) signaling in the neurogenic niche of the hippocampus, analogous to the previously demonstrated changes in the myogenic niche of skeletal muscle with age. Exploring the hypothesis that youthful calibration of key signaling pathways may enhance regeneration of multiple old tissues, we found that systemically attenuating TGF-β signaling with a single drug simultaneously enhanced neurogenesis and muscle regeneration in the same old mice, findings further substantiated via genetic perturbations. At the levels of cellular mechanism, our results establish that the age-specific increase in TGF-β1 in the stem cell niches of aged hippocampus involves microglia and that such an increase is pro-inflammatory both in brain and muscle, as assayed by the elevated expression of β2 microglobulin (B2M), a component of MHC class I molecules. These findings suggest that at high levels typical of aged tissues, TGF-β1 promotes inflammation instead of its canonical role in attenuating immune responses. In agreement with this conclusion, inhibition of TGF-β1 signaling normalized B2M to young levels in both studied tissues. | 26003168
 |
Analysing human neural stem cell ontogeny by consecutive isolation of Notch active neural progenitors.
Edri, R; Yaffe, Y; Ziller, MJ; Mutukula, N; Volkman, R; David, E; Jacob-Hirsch, J; Malcov, H; Levy, C; Rechavi, G; Gat-Viks, I; Meissner, A; Elkabetz, Y
Nature communications
6
6500
2015
Afficher le résumé
Decoding heterogeneity of pluripotent stem cell (PSC)-derived neural progeny is fundamental for revealing the origin of diverse progenitors, for defining their lineages, and for identifying fate determinants driving transition through distinct potencies. Here we have prospectively isolated consecutively appearing PSC-derived primary progenitors based on their Notch activation state. We first isolate early neuroepithelial cells and show their broad Notch-dependent developmental and proliferative potential. Neuroepithelial cells further yield successive Notch-dependent functional primary progenitors, from early and midneurogenic radial glia and their derived basal progenitors, to gliogenic radial glia and adult-like neural progenitors, together recapitulating hallmarks of neural stem cell (NSC) ontogeny. Gene expression profiling reveals dynamic stage-specific transcriptional patterns that may link development of distinct progenitor identities through Notch activation. Our observations provide a platform for characterization and manipulation of distinct progenitor cell types amenable for developing streamlined neural lineage specification paradigms for modelling development in health and disease. | 25799239
 |
Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline.
Cho, KO; Lybrand, ZR; Ito, N; Brulet, R; Tafacory, F; Zhang, L; Good, L; Ure, K; Kernie, SG; Birnbaum, SG; Scharfman, HE; Eisch, AJ; Hsieh, J
Nature communications
6
6606
2015
Afficher le résumé
Acute seizures after a severe brain insult can often lead to epilepsy and cognitive impairment. Aberrant hippocampal neurogenesis follows the insult but the role of adult-generated neurons in the development of chronic seizures or associated cognitive deficits remains to be determined. Here we show that the ablation of adult neurogenesis before pilocarpine-induced acute seizures in mice leads to a reduction in chronic seizure frequency. We also show that ablation of neurogenesis normalizes epilepsy-associated cognitive deficits. Remarkably, the effect of ablating adult neurogenesis before acute seizures is long lasting as it suppresses chronic seizure frequency for nearly 1 year. These findings establish a key role of neurogenesis in chronic seizure development and associated memory impairment and suggest that targeting aberrant hippocampal neurogenesis may reduce recurrent seizures and restore cognitive function following a pro-epileptic brain insult. | 25808087
 |
Identification of distinct ChAT⁺ neurons and activity-dependent control of postnatal SVZ neurogenesis.
Paez-Gonzalez, P; Asrican, B; Rodriguez, E; Kuo, CT
Nature neuroscience
17
934-42
2014
Afficher le résumé
Postnatal and adult subventricular zone (SVZ) neurogenesis is believed to be primarily controlled by neural stem cell (NSC)-intrinsic mechanisms, interacting with extracellular and niche-driven cues. Although behavioral experiments and disease states have suggested possibilities for higher level inputs, it is unknown whether neural activity patterns from discrete circuits can directly regulate SVZ neurogenesis. We identified a previously unknown population of choline acetyltransferase (ChAT)(+) neurons residing in the rodent SVZ neurogenic niche. These neurons showed morphological and functional differences from neighboring striatal counterparts and released acetylcholine locally in an activity-dependent fashion. Optogenetic inhibition and stimulation of subependymal ChAT(+) neurons in vivo indicated that they were necessary and sufficient to control neurogenic proliferation. Furthermore, whole-cell recordings and biochemical experiments revealed direct SVZ NSC responses to local acetylcholine release, synergizing with fibroblast growth factor receptor activation to increase neuroblast production. These results reveal an unknown gateway connecting SVZ neurogenesis to neuronal activity-dependent control and suggest possibilities for modulating neuroregenerative capacities in health and disease. | 24880216
 |
Analysis of Mll1 deficiency identifies neurogenic transcriptional modules and Brn4 as a factor for direct astrocyte-to-neuron reprogramming.
Potts, MB; Siu, JJ; Price, JD; Salinas, RD; Cho, MJ; Ramos, AD; Hahn, J; Margeta, M; Oldham, MC; Lim, DA
Neurosurgery
75
472-82; discussion 482
2014
Afficher le résumé
Mixed lineage leukemia-1 (Mll1) epigenetically regulates gene expression patterns that specify cellular identity in both embryonic development and adult stem cell populations. In the adult mouse brain, multipotent neural stem cells (NSCs) in the subventricular zone generate new neurons throughout life, and Mll1 is required for this postnatal neurogenesis but not for glial cell differentiation. Analysis of Mll1-dependent transcription may identify neurogenic genes useful for the direct reprogramming of astrocytes into neurons.To identify Mll1-dependent transcriptional modules and to determine whether genes in the neurogenic modules can be used to directly reprogram astrocytes into neurons.We performed gene coexpression module analysis on microarray data from differentiating wild-type and Mll1-deleted subventricular zone NSCs. Key developmental regulators belonging to the neurogenic modules were overexpressed in Mll1-deleted cells and cultured cortical astrocytes, and cell phenotypes were analyzed by immunocytochemistry and electrophysiology.Transcriptional modules that correspond to neurogenesis were identified in wild-type NSCs. Modules related to astrocytes and oligodendrocytes were enriched in Mll1-deleted NSCs, consistent with their gliogenic potential. Overexpression of genes selected from the neurogenic modules enhanced the production of neurons from Mll1-deleted cells, and overexpression of Brn4 (Pou3f4) in nonneurogenic cortical astroglia induced their transdifferentiation into electrophysiologically active neurons.Our results demonstrate that Mll1 is required for the expression of neurogenic but not gliogenic transcriptional modules in a multipotent NSC population and further indicate that specific Mll1-dependent genes may be useful for direct reprogramming strategies. | 24887289
 |
Onecut1 is essential for horizontal cell genesis and retinal integrity.
Wu, F; Li, R; Umino, Y; Kaczynski, TJ; Sapkota, D; Li, S; Xiang, M; Fliesler, SJ; Sherry, DM; Gannon, M; Solessio, E; Mu, X
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
13053-65, 13065a
2013
Afficher le résumé
Horizontal cells are interneurons that synapse with photoreceptors in the outer retina. Their genesis during development is subject to regulation by transcription factors in a hierarchical manner. Previously, we showed that Onecut 1 (Oc1), an atypical homeodomain transcription factor, is expressed in developing horizontal cells (HCs) and retinal ganglion cells (RGCs) in the mouse retina. Herein, by knocking out Oc1 specifically in the developing retina, we show that the majority (∼80%) of HCs fail to form during early retinal development, implying that Oc1 is essential for HC genesis. However, no other retinal cell types, including RGCs, were affected in the Oc1 knock-out. Analysis of the genetic relationship between Oc1 and other transcription factor genes required for HC development revealed that Oc1 functions downstream of FoxN4, in parallel with Ptf1a, but upstream of Lim1 and Prox1. By in utero electroporation, we found that Oc1 and Ptf1a together are not only essential, but also sufficient for determination of HC fate. In addition, the synaptic connections in the outer plexiform layer are defective in Oc1-null mice, and photoreceptors undergo age-dependent degeneration, indicating that HCs are not only an integral part of the retinal circuitry, but also are essential for the survival of photoreceptors. In sum, these results demonstrate that Oc1 is a critical determinant of HC fate, and reveal that HCs are essential for photoreceptor viability, retinal integrity, and normal visual function. | 23926259
 |
Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain.
Park, JH; Glass, Z; Sayed, K; Michurina, TV; Lazutkin, A; Mineyeva, O; Velmeshev, D; Ward, WF; Richardson, A; Enikolopov, G
The European journal of neuroscience
37
1987-93
2013
Afficher le résumé
Production of new neurons from stem cells is important for cognitive function, and the reduction of neurogenesis in the aging brain may contribute to the accumulation of age-related cognitive deficits. Restriction of calorie intake and prolonged treatment with rapamycin have been shown to extend the lifespan of animals and delay the onset of the age-related decline in tissue and organ function. Using a reporter line in which neural stem and progenitor cells are marked by the expression of green fluorescent protein (GFP), we examined the effect of prolonged exposure to calorie restriction (CR) or rapamycin on hippocampal neural stem and progenitor cell proliferation in aging mice. We showed that CR increased the number of dividing cells in the dentate gyrus of female mice. The majority of these cells corresponded to nestin-GFP-expressing neural stem or progenitor cells; however, this increased proliferative activity of stem and progenitor cells did not result in a significant increase in the number of doublecortin-positive newborn neurons. Our results suggest that restricted calorie intake may increase the number of divisions that neural stem and progenitor cells undergo in the aging brain of females. | 23773068
 |
EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons.
Puehringer, D; Orel, N; Lüningschrör, P; Subramanian, N; Herrmann, T; Chao, MV; Sendtner, M
Nature neuroscience
16
407-15
2013
Afficher le résumé
The development of neuronal networks in the neocortex depends on control mechanisms for mitosis and migration that allow newborn neurons to find their accurate position. Multiple mitogens, neurotrophic factors, guidance molecules and their corresponding receptors are involved in this process, but the mechanisms by which these signals are integrated are only poorly understood. We found that TrkB and TrkC, the receptors for brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), are activated by epidermal growth factor receptor (EGFR) signaling rather than by BDNF or NT-3 in embryonic mouse cortical precursor cells. This transactivation event regulated migration of early neuronal cells to their final position in the developing cortex. Transactivation by EGF led to membrane translocation of TrkB, promoting its signaling responsiveness. Our results provide genetic evidence that TrkB and TrkC activation in early cortical neurons do not depend on BDNF and NT-3, but instead on transactivation by EGFR signaling. | 23416450
 |
TGF-β superfamily gene expression and induction of the Runx1 transcription factor in adult neurogenic regions after brain injury.
Logan, TT; Villapol, S; Symes, AJ
PloS one
8
e59250
2013
Afficher le résumé
Traumatic brain injury (TBI) increases neurogenesis in the forebrain subventricular zone (SVZ) and the hippocampal dentate gyrus (DG). Transforming growth factor-β (TGF-β) superfamily cytokines are important regulators of adult neurogenesis, but their involvement in the regulation of this process after brain injury is unclear. We subjected adult mice to controlled cortical impact (CCI) injury, and isolated RNA from the SVZ and DG at different post-injury time points. qPCR array analysis showed that cortical injury caused significant alterations in the mRNA expression of components and targets of the TGF-β, BMP, and activin signaling pathways in the SVZ and DG after injury, suggesting that these pathways could regulate post-injury neurogenesis. In both neurogenic regions, the injury also induced expression of Runt-related transcription factor-1 (Runx1), which can interact with intracellular TGF-β Smad signaling pathways. CCI injury strongly induced Runx1 expression in activated and proliferating microglial cells throughout the neurogenic regions. Runx1 protein was also expressed in a subset of Nestin- and GFAP-expressing putative neural stem or progenitor cells in the DG and SVZ after injury. In the DG only, these Runx1+ progenitors proliferated. Our data suggest potential roles for Runx1 in the processes of microglial cell activation and proliferation and in neural stem cell proliferation after TBI. | 23555640
 |