Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area.
Koo, JW; Mazei-Robison, MS; LaPlant, Q; Egervari, G; Braunscheidel, KM; Adank, DN; Ferguson, D; Feng, J; Sun, H; Scobie, KN; Damez-Werno, DM; Ribeiro, E; Peña, CJ; Walker, D; Bagot, RC; Cahill, ME; Anderson, SA; Labonté, B; Hodes, GE; Browne, H; Chadwick, B; Robison, AJ; Vialou, VF; Dias, C; Lorsch, Z; Mouzon, E; Lobo, MK; Dietz, DM; Russo, SJ; Neve, RL; Hurd, YL; Nestler, EJ
Nature neuroscience
18
415-22
2015
Afficher le résumé
Brain-derived neurotrophic factor (BDNF) has a crucial role in modulating neural and behavioral plasticity to drugs of abuse. We found a persistent downregulation of exon-specific Bdnf expression in the ventral tegmental area (VTA) in response to chronic opiate exposure, which was mediated by specific epigenetic modifications at the corresponding Bdnf gene promoters. Exposure to chronic morphine increased stalling of RNA polymerase II at these Bdnf promoters in VTA and altered permissive and repressive histone modifications and occupancy of their regulatory proteins at the specific promoters. Furthermore, we found that morphine suppressed binding of phospho-CREB (cAMP response element binding protein) to Bdnf promoters in VTA, which resulted from enrichment of trimethylated H3K27 at the promoters, and that decreased NURR1 (nuclear receptor related-1) expression also contributed to Bdnf repression and associated behavioral plasticity to morphine. Our findings suggest previously unknown epigenetic mechanisms of morphine-induced molecular and behavioral neuroadaptations. | | | 25643298
 |
Imaging activity in neurons and glia with a Polr2a-based and cre-dependent GCaMP5G-IRES-tdTomato reporter mouse.
Gee, JM; Smith, NA; Fernandez, FR; Economo, MN; Brunert, D; Rothermel, M; Morris, SC; Talbot, A; Palumbos, S; Ichida, JM; Shepherd, JD; West, PJ; Wachowiak, M; Capecchi, MR; Wilcox, KS; White, JA; Tvrdik, P
Neuron
83
1058-72
2014
Afficher le résumé
New strategies for introducing genetically encoded activity indicators into animal models facilitate the investigation of nervous system function. We have developed the PC::G5-tdT mouse line that expresses the GCaMP5G calcium indicator in a Cre-dependent fashion. Instead of targeting the ROSA26 locus, we inserted the reporter cassette nearby the ubiquitously expressed Polr2a gene without disrupting locus integrity. The indicator was tagged with IRES-tdTomato to aid detection of positive cells. This reporter system is effective in a wide range of developmental and cellular contexts. We recorded spontaneous cortical calcium waves in intact awake newborns and evaluated concentration-dependent responses to odorants in the adult olfactory bulb. Moreover, PC::G5-tdT effectively reports intracellular calcium dynamics in somas and fine processes of astrocytes and microglial cells. Through electrophysiological and behavioral analyses, we determined that GCaMP5G expression had no major impact on nervous system performance. PC::G5-tdT will be instrumental for a variety of brain mapping experiments. | | | 25155958
 |
Cerebellar cortical lamination and foliation require cyclin A2.
Otero, JJ; Kalaszczynska, I; Michowski, W; Wong, M; Gygli, PE; Gokozan, HN; Griveau, A; Odajima, J; Czeisler, C; Catacutan, FP; Murnen, A; Schüller, U; Sicinski, P; Rowitch, D
Developmental biology
385
328-39
2014
Afficher le résumé
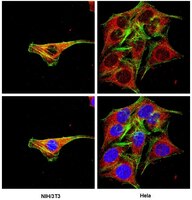
The mammalian genome encodes two A-type cyclins, which are considered potentially redundant yet essential regulators of the cell cycle. Here, we tested requirements for cyclin A1 and cyclin A2 function in cerebellar development. Compound conditional loss of cyclin A1/A2 in neural progenitors resulted in severe cerebellar hypoplasia, decreased proliferation of cerebellar granule neuron progenitors (CGNP), and Purkinje (PC) neuron dyslamination. Deletion of cyclin A2 alone showed an identical phenotype, demonstrating that cyclin A1 does not compensate for cyclin A2 loss in neural progenitors. Cyclin A2 loss lead to increased apoptosis at early embryonic time points but not at post-natal time points. In contrast, neural progenitors of the VZ/SVZ did not undergo increased apoptosis, indicating that VZ/SVZ-derived and rhombic lip-derived progenitor cells show differential requirements to cyclin A2. Conditional knockout of cyclin A2 or the SHH proliferative target Nmyc in CGNP also resulted in PC neuron dyslamination. Although cyclin E1 has been reported to compensate for cyclin A2 function in fibroblasts and is upregulated in cyclin A2 null cerebella, cyclin E1 expression was unable to compensate for loss-of cyclin A2 function. | Western Blotting | | 24184637
 |
Extrasynaptic GABAA receptors in mediodorsal thalamic nucleus modulate fear extinction learning.
Paydar, A; Lee, B; Gangadharan, G; Lee, S; Hwang, EM; Shin, HS
Molecular brain
7
39
2014
Afficher le résumé
The gamma-amino-butyric acid (GABA) system is a critical mediator of fear extinction process. GABA can induce "phasic" or "tonic" inhibition in neurons through synaptic or extrasynaptic GABAA receptors, respectively. However, role of the thalamic "tonic GABA inhibition" in cognition has not been explored. We addressed this issue in extinction of conditioned fear in mice.Here, we show that GABAA receptors in the mediodorsal thalamic nucleus (MD) modulate fear extinction. Microinjection of gabazine, a GABAA receptor antagonist, into the MD decreased freezing behavior in response to the conditioned stimulus and thus facilitated fear extinction. Interestingly, microinjection of THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol), a preferential agonist for the δ-subunit of extrasynaptic GABAA receptors, into the MD attenuated fear extinction. In the opposite direction, an MD-specific knock-out of the extrasynaptic GABAA receptors facilitated fear extinction.Our results suggest that "tonic GABA inhibition" mediated by extrasynaptic GABAA receptors in MD neurons, suppresses fear extinction learning. These results raise a possibility that pharmacological control of tonic mode of GABAA receptor activation may be a target for treatment of anxiety disorders like post-traumatic stress disorder. | Immunohistochemistry | | 24886120
 |
G9a influences neuronal subtype specification in striatum.
Maze, I; Chaudhury, D; Dietz, DM; Von Schimmelmann, M; Kennedy, PJ; Lobo, MK; Sillivan, SE; Miller, ML; Bagot, RC; Sun, H; Turecki, G; Neve, RL; Hurd, YL; Shen, L; Han, MH; Schaefer, A; Nestler, EJ
Nature neuroscience
17
533-9
2014
Afficher le résumé
Cocaine-mediated repression of the histone methyltransferase (HMT) G9a has recently been implicated in transcriptional, morphological and behavioral responses to chronic cocaine administration. Here, using a ribosomal affinity purification approach, we found that G9a repression by cocaine occurred in both Drd1-expressing (striatonigral) and Drd2-expressing (striatopallidal) medium spiny neurons. Conditional knockout and overexpression of G9a within these distinct cell types, however, revealed divergent behavioral phenotypes in response to repeated cocaine treatment. Our studies further indicated that such developmental deletion of G9a selectively in Drd2 neurons resulted in the unsilencing of transcriptional programs normally specific to striatonigral neurons and in the acquisition of Drd1-associated projection and electrophysiological properties. This partial striatopallidal to striatonigral 'switching' phenotype in mice indicates a new role for G9a in contributing to neuronal subtype identity and suggests a critical function for cell type-specific histone methylation patterns in the regulation of behavioral responses to environmental stimuli. | Immunohistochemistry | | 24584053
 |
Germ-line recombination activity of the widely used hGFAP-Cre and nestin-Cre transgenes.
Zhang, J; Dublin, P; Griemsmann, S; Klein, A; Brehm, R; Bedner, P; Fleischmann, BK; Steinhäuser, C; Theis, M
PloS one
8
e82818
2013
Afficher le résumé
Herein we demonstrate with PCR, immunodetection and reporter gene approaches that the widely used human Glial Fibrillary Acidic Protein (hGFAP)-Cre transgene exhibits spontaneous germ-line recombination activity in leading to deletion in brain, heart and tail tissue with high frequency. The ectopic activity of hGFAP-Cre requires a rigorous control. We likewise observed that a second widely used nestin-Cre transgene shows germ-line deletion. Here we describe procedures to identify mice with germ-line recombination mediated by the hGFAP-Cre and nestin-Cre transgenes. Such control is essential to avoid pleiotropic effects due to germ-line deletion of loxP-flanked target genes and to maintain the CNS-restricted deletion status in transgenic mouse colonies. | | | 24349371
 |
Axon position within the corpus callosum determines contralateral cortical projection.
Zhou, J; Wen, Y; She, L; Sui, YN; Liu, L; Richards, LJ; Poo, MM
Proceedings of the National Academy of Sciences of the United States of America
110
E2714-23
2013
Afficher le résumé
How developing axons in the corpus callosum (CC) achieve their homotopic projection to the contralateral cortex remains unclear. We found that axonal position within the CC plays a critical role in this projection. Labeling of nearby callosal axons in mice showed that callosal axons were segregated in an orderly fashion, with those from more medial cerebral cortex located more dorsally and subsequently projecting to more medial contralateral cortical regions. The normal axonal order within the CC was grossly disturbed when semaphorin3A/neuropilin-1 signaling was disrupted. However, the order in which axons were positioned within the CC still determined their contralateral projection, causing a severe disruption of the homotopic contralateral projection that persisted at postnatal day 30, when the normal developmental refinement of contralateral projections is completed in wild-type (WT) mice. Thus, the orderly positioning of axons within the CC is a primary determinant of how homotopic interhemispheric projections form in the contralateral cortex. | | | 23812756
 |
Neuronal cadherin (NCAD) increases sensory neurite formation and outgrowth on astrocytes.
Ferguson, TA; Scherer, SS
Neuroscience letters
522
108-12
2011
Afficher le résumé
We examined the neurite outgrowth of sensory neurons on astrocytes following the genetic deletion of N-cadherin (NCAD). Deletion abolished immunostaining for NCAD and the other classical cadherins, indicating that NCAD is likely the only classical cadherin expressed by astrocytes. Only 38% of neurons grown on NCAD-deficient astrocytes for 24 h produced neurites, as compared to 74% of neurons grown on NCAD-expressing astrocytes. Of the neurons that produced neurites, those grown on NCAD-deficient astrocytes had a mean total length of 378 μm, as compared to 1093 μm for neurons grown on NCAD-expressing astrocytes. Thus, the loss of NCAD greatly impairs the formation and extension neurites on astrocytes. | | | 22698587
 |
Fusion of intestinal epithelial cells with bone marrow derived cells is dispensable for tissue homeostasis.
de Jong, JH; Rodermond, HM; Zimberlin, CD; Lascano, V; De Sousa E Melo, F; Richel, DJ; Medema, JP; Vermeulen, L
Scientific reports
2
271
2011
Afficher le résumé
The epithelial lining of the intestine is characterized by an immense cellular turn-over ascertaining an extensive regenerative capacity. Multiple reports suggest that besides the local intestinal stem cell pool, circulating cells of bone marrow origin (BMDCs) contribute to this process by fusing with the epithelial lineage. However, the functional relevance of these observations is unknown. In the present study we employ a model system in which we cannot only detect cell fusion but also examine the functional importance of this process in vivo. Our results indicate that fusion between BMDCs and intestinal epithelial cells is an extremely rare event under physiological conditions. More importantly, by employing a system in which fusion-derived cells can be specifically deleted after extensive tissue damage, we present evidence that cell fusion is not relevant for tissue regeneration. Our data decisively demonstrates that intestinal epithelial homeostasis and regeneration is not dependent on cell fusion involving BMDCs. | | | 22355783
 |
Specific β-containing integrins exert differential control on proliferation and two-dimensional collective cell migration in mammary epithelial cells.
Jeanes, AI; Wang, P; Moreno-Layseca, P; Paul, N; Cheung, J; Tsang, R; Akhtar, N; Foster, FM; Brennan, K; Streuli, CH
The Journal of biological chemistry
287
24103-12
2011
Afficher le résumé
Understanding how cell cycle is regulated in normal mammary epithelia is essential for deciphering defects of breast cancer and therefore for developing new therapies. Signals provided by both the extracellular matrix and growth factors are essential for epithelial cell proliferation. However, the mechanisms by which adhesion controls cell cycle in normal epithelia are poorly established. In this study, we describe the consequences of removing the β1-integrin gene from primary cultures of mammary epithelial cells in situ, using CreER. Upon β1-integrin gene deletion, the cells were unable to progress efficiently through S-phase, but were still able to undergo collective two-dimensional migration. These responses are explained by the presence of β3-integrin in β1-integrin-null cells, indicating that integrins containing different β-subunits exert differential control on mammary epithelial proliferation and migration. β1-Integrin deletion did not inhibit growth factor signaling to Erk or prevent the recruitment of core adhesome components to focal adhesions. Instead the S-phase arrest resulted from defective Rac activation and Erk translocation to the nucleus. Rac inhibition prevented Erk translocation and blocked proliferation. Activated Rac1 rescued the proliferation defect in β1-integrin-depleted cells, indicating that this GTPase is essential in propagating proliferative β1-integrin signals. These results show that β1-integrins promote cell cycle in mammary epithelial cells, whereas β3-integrins are involved in migration. | Immunofluorescence | | 22511753
 |