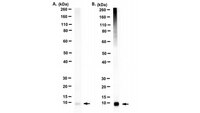
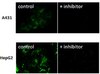
Distinct regulatory ribosomal ubiquitylation events are reversible and hierarchically organized
Danielle M Garshott 1 , Elayanambi Sundaramoorthy 1 , Marilyn Leonard 1 , Eric J Bennett
Elife
9
e54023
2020
Show Abstract
Activation of the integrated stress response (ISR) or the ribosome-associated quality control (RQC) pathway stimulates regulatory ribosomal ubiquitylation (RRub) on distinct 40S ribosomal proteins, yet the cellular role and fate of ubiquitylated proteins remain unclear. We demonstrate that uS10 and uS5 ubiquitylation are dependent upon eS10 or uS3 ubiquitylation, respectively, suggesting that a hierarchical relationship exists among RRub events establishing a ubiquitin code on ribosomes. We show that stress dependent RRub events diminish after initial stimuli and that demodification by deubiquitylating enzymes contributes to reduced RRub levels during stress recovery. Utilizing an optical RQC reporter we identify OTUD3 and USP21 as deubiquitylating enzymes that antagonize ZNF598-mediated 40S ubiquitylation and can limit RQC activation. Critically, cells lacking USP21 or OTUD3 have altered RQC activity and delayed eS10 deubiquitylation indicating a functional role for deubiquitylating enzymes within the RQC pathway. | 32011234
 |
Structure of E3 ligase E6AP with a proteasome-binding site provided by substrate receptor hRpn10
Gwen R Buel 1 , Xiang Chen 2 , Raj Chari 3 , Maura J O'Neill 4 , Danielle L Ebelle 1 , Conor Jenkins 4 , Vinidhra Sridharan 1 , Sergey G Tarasov 5 , Nadya I Tarasova 6 , Thorkell Andresson 4 , Kylie J Walters 7
Nat Commun
11(1)
1291
2020
Show Abstract
Regulated proteolysis by proteasomes involves ~800 enzymes for substrate modification with ubiquitin, including ~600 E3 ligases. We report here that E6AP/UBE3A is distinguished from other E3 ligases by having a 12 nM binding site at the proteasome contributed by substrate receptor hRpn10/PSMD4/S5a. Intrinsically disordered by itself, and previously uncharacterized, the E6AP-binding domain in hRpn10 locks into a well-defined helical structure to form an intermolecular 4-helix bundle with the E6AP AZUL, which is unique to this E3. We thus name the hRpn10 AZUL-binding domain RAZUL. We further find in human cells that loss of RAZUL by CRISPR-based gene editing leads to loss of E6AP at proteasomes. Moreover, proteasome-associated ubiquitin is reduced following E6AP knockdown or displacement from proteasomes, suggesting that E6AP ubiquitinates substrates at or for the proteasome. Altogether, our findings indicate E6AP to be a privileged E3 for the proteasome, with a dedicated, high affinity binding site contributed by hRpn10. | 32157086
 |
Major vault protein suppresses obesity and atherosclerosis through inhibiting IKK-NF-κB signaling mediated inflammation
Jingjing Ben 1 , Bin Jiang 2 , Dongdong Wang 2 , Qingling Liu 2 , Yongjing Zhang 2 , Yu Qi 2 , Xing Tong 2 , Lili Chen 2 , Xianzhong Liu 3 , Yan Zhang 2 , Xudong Zhu 2 , Xiaoyu Li 2 , Hanwen Zhang 2 , Hui Bai 2 , Qing Yang 2 , Junqing Ma 2 , Erik A C Wiemer 4 , Yong Xu 2 , Qi Chen
Nat Commun
10(1)
1801
2019
Show Abstract
Macrophage-orchestrated, low-grade chronic inflammation plays a pivotal role in obesity and atherogenesis. However, the underlying regulatory mechanisms remain incompletely understood. Here, we identify major vault protein (MVP), the main component of unique cellular ribonucleoprotein particles, as a suppressor for NF-κB signaling in macrophages. Both global and myeloid-specific MVP gene knockout aggravates high-fat diet induced obesity, insulin resistance, hepatic steatosis and atherosclerosis in mice. The exacerbated metabolic disorders caused by MVP deficiency are accompanied with increased macrophage infiltration and heightened inflammatory responses in the microenvironments. In vitro studies reveal that MVP interacts with TRAF6 preventing its recruitment to IRAK1 and subsequent oligomerization and ubiquitination. Overexpression of MVP and its α-helical domain inhibits the activity of TRAF6 and suppresses macrophage inflammation. Our results demonstrate that macrophage MVP constitutes a key constraint of NF-κB signaling thereby suppressing metabolic diseases. | 30996248
 |
Active Protein Neddylation or Ubiquitylation Is Dispensable for Stress Granule Dynamics
Sebastian Markmiller 1 , Amit Fulzele 2 , Reneé Higgins 2 , Marilyn Leonard 2 , Gene W Yeo 3 , Eric J Bennett
Cell Rep
27(5)
1356-1363
2019
Show Abstract
Stress granule (SG) formation is frequently accompanied by ubiquitin proteasome system (UPS) impairment and ubiquitylated protein accumulation. SGs, ubiquitin, and UPS components co-localize, but the relationship between the ubiquitin pathway and SGs has not been systematically characterized. We utilize pharmacological inhibition of either the ubiquitin- or NEDD8-activating enzyme (UAE or NAE) to probe whether active ubiquitylation or neddylation modulate SG dynamics. We show that UAE inhibition results in rapid loss of global protein ubiquitylation using ubiquitin-specific proteomics. Critically, inhibiting neither UAE nor NAE significantly affected SG formation or disassembly, indicating that active protein ubiquitylation or neddylation is dispensable for SG dynamics. Using antibodies with varying preference for free ubiquitin or polyubiquitin and fluorescently tagged ubiquitin variants in combination with UAE inhibition, we show that SGs co-localize primarily with unconjugated ubiquitin rather than polyubiquitylated proteins. These findings clarify the role of ubiquitin in SG biology and suggest that free ubiquitin may alter SG protein interactions. | 31042464
 |
Early feeding of rainbow trout ( Oncorhynchus mykiss) with methionine-deficient diet over a 2 week period: consequences for liver mitochondria in juveniles
Sarah Séité 1 2 3 , Karthik Masagounder 3 , Cécile Heraud 1 , Vincent Véron 1 , Lucie Marandel 1 , Stéphane Panserat 1 , Iban Seiliez
J Exp Biol
222(Pt 18)
jeb203687
2019
Show Abstract
Methionine is a key factor in modulating the cellular availability of the main biological methyl donor S-adenosylmethionine (SAM), which is required for all biological methylation reactions including DNA and histone methylation. As such, it represents a potential critical factor in nutritional programming. Here, we investigated whether early methionine restriction at first feeding could have long-term programmed metabolic consequences in rainbow trout. For this purpose, trout fry were fed with either a control diet (C) or a methionine-deficient diet (MD) for 2 weeks from the first exogenous feeding. Next, fish were subjected to a 5 month growth trial with a standard diet followed by a 2 week challenge (with the MD or C diet) to test the programming effect of the early methionine restriction. The results showed that, whatever the dietary treatment of fry, the 2 week challenge with the MD diet led to a general mitochondrial defect associated with an increase in endoplasmic reticulum stress, mitophagy and apoptosis, highlighting the existence of complex cross-talk between these different functions. Moreover, for the first time, we also observed that fish fed the MD diet at the first meal later exhibited an increase in several critical factors of mitophagy, hinting that the early nutritional stimulus with methionine deficiency resulted in long-term programming of this cell function. Together, these data extend our understanding of the role of dietary methionine and emphasize the potential for this amino acid in the application of new feeding strategies, such as nutritional programming, to optimize the nutrition and health of farmed fish. | 31488624
 |
Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations
Keith A Josephs 1 , Zeshan Ahmed, Omi Katsuse, Joseph F Parisi, Bradley F Boeve, David S Knopman, Ronald C Petersen, Peter Davies, Ranjan Duara, Neill R Graff-Radford, Ryan J Uitti, Rosa Rademakers, Jennifer Adamson, Matthew Baker, Michael L Hutton, Dennis W Dickson
J Neropathol Exp Neurol
66(2)
142-51
2007
Show Abstract
Frontotemporal lobar degeneration is heterogeneous; cases with tau- and synuclein-negative, ubiquitin-positive neuronal inclusions are the most common, and some have mutations in the gene for progranulin (PGRN). The purpose of this study was to determine whether there were distinctive clinical and neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with PGRN mutations. A retrospective review of medical records and semiquantitative neuropathologic analysis was performed on 18 PGRN(+) and 24 PGRN(-) cases. Clinically, PGRN(+) cases had more frequent language impairment and parkinsonism. Pathologically, PGRN(+) cases had smaller brains, more marked global atrophy, and more frontal atrophy. There was no difference in the frequency of hippocampal sclerosis. The pathology of PGRN(+) cases was relatively homogeneous, whereas PGRN(-) cases were more heterogenous. PGRN(+) cases had greater density of cortical ubiquitin-immunoreactive lesions, especially dystrophic neurites in layer II. Intranuclear inclusions were present in all PGRN(+) and 42% of PGRN(-) cases. The results suggest that frontotemporal lobar degeneration with ubiquitin-positive inclusions due to PGRN mutations has several characteristic features, including ubiquitin-immunoreactive neuritic pathology in superficial cortical layers and neuronal intranuclear inclusions. On the other hand, there is no histopathologic feature or combination of features that is pathognomonic. Neuronal intranuclear inclusions are virtually always present, but they can be detected in PGRN(-) cases. | 17278999
 |