ABN1650 Sigma-AldrichAnti-Aβ-42 Antibody, oligomeric (VIA)
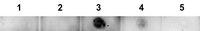
Anti-Aβ-42, oligomeric (VIA), Cat. No. ABN1650, is a highly specific rabbit polyclonal antibody that targets oligomeric amyloid beta 42 and has been tested in Dot Blot, Immunofluorescence, Immunohistochemistry, and Western Blotting.
More>> Anti-Aβ-42, oligomeric (VIA), Cat. No. ABN1650, is a highly specific rabbit polyclonal antibody that targets oligomeric amyloid beta 42 and has been tested in Dot Blot, Immunofluorescence, Immunohistochemistry, and Western Blotting. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Species Reactivity | Key Applications | Host | Format | Antibody Type |
|---|---|---|---|---|
| H | DB, IF, IHC, WB | Rb | Serum | Polyclonal Antibody |
| References |
|---|
| Product Information | |
|---|---|
| Format | Serum |
| Presentation | Rabbit polyclonal antibody serum with 0.05% sodium azide. |
| Quality Level | MQ100 |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 100 µL |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| ABN1650 | 04054839092022 |
Documentation
Anti-Aβ-42 Antibody, oligomeric (VIA) SDS
| Title |
|---|
Anti-Aβ-42 Antibody, oligomeric (VIA) Certificates of Analysis
| Title | Lot Number |
|---|---|
| Anti-Aβ-42, oligomeric (VIA) - 3239831 | 3239831 |
| Anti-Aβ-42, oligomeric (VIA) -Q2774381 | Q2774381 |
| Anti-Aβ-42, oligomeric (VIA) Polyclonal Antibody | 3775380 |