567616 Sigma-AldrichSodium 4-Phenylbutyrate - CAS 1716-12-7 - Calbiochem
Sodium 4-Phenylbutyrate, CAS 1716-12-7, is a novel anti-neoplastic agent and transcriptional regulator. Also acts as an inducer of tumor cytostasis and differentiation.
More>> Sodium 4-Phenylbutyrate, CAS 1716-12-7, is a novel anti-neoplastic agent and transcriptional regulator. Also acts as an inducer of tumor cytostasis and differentiation. Less<<Synonyme: SPB, PBNa, 4PBA, 4-Phenylbutyrate, Na, BDK Inhibitor I, BCKD Kinase Inhibitor I, Branched-Chain α-Ketoacid Dehydrogenase Kinase Inhibitor I
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
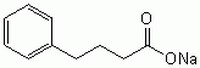
| 1716-12-7 | C₁₀H₁₁O₂Na |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 567616-100MG |
|
Kst.-Ampulle | 100 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 1716-12-7 |
| ATP Competitive | N |
| Form | White to slightly yellow solid |
| Hill Formula | C₁₀H₁₁O₂Na |
| Chemical formula | C₁₀H₁₁O₂Na |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | Sodium 4-Phenylbutyrate, CAS 1716-12-7, is a novel anti-neoplastic agent and transcriptional regulator. Also acts as an inducer of tumor cytostasis and differentiation. |
| Biological Information | |
|---|---|
| Primary Target | Anti-neoplastic agent |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 567616-100MG | 04055977266818 |
Documentation
Sodium 4-Phenylbutyrate - CAS 1716-12-7 - Calbiochem SDB
| Titel |
|---|
Sodium 4-Phenylbutyrate - CAS 1716-12-7 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 567616 |
Literatur
| Übersicht |
|---|
| Tso, S.C., et al. 2013. Proc. Natl. Acad. Sci. USA 110, 9728. Ozcan, U., et al. 2006. Science 313, 1137. Carducci, M.A., et al. 2001. Clin. Cancer Res. 7, 3047. Rubenstein, R.C. and Zeitlin, P.L. 2000. Am.J. Physiol. Cell. Physiol. 278, C259. Lea, M.A., et al. 1999. Anticancer Res. 19, 1971. Miller, A.C., et al. 1997. Int. J. Radiat. Biol. 72, 211. Rubenstein, R.C., et al. 1997. J. Clin. Invest. 100, 2457. Pineau, T., et al. 1996. Biochem. Pharmacol. 52, 659. Collins, A.F., et al. 1995. Blood 85, 43. Lea, M.A., et al. 1995. Anticancer Res. 15, 879. Figg, W.D., et al. 1994. Anticancer Drugs 5, 336. Liu, L., et al. 1994. J. Invest. Dermatol. 103, 335. |
Broschüre
| Titel |
|---|
| Caspases and other Apoptosis Related Tools Brochure |