539225 Sigma-AldrichProtein Disulfide Isomerase Inhibitor III, PACMA 31 - Calbiochem
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 539225-25MG |
|
Glasflasche | 25 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable propynoic acid carbamoyl methyl amide (PACMA) that is more potent than PAO (Cat. No. 521000) in inhibiting PDI (protein disulfide isomerase) thioloxidoreductase activity (IC50 = 10 and 85 µM, respectively; 1h preincubation) by altering PDI secondary conformation via covalent modification of active site C(397)GHC(400) motif cysteines. Shown to inhibit the proliferation of human ovarian cancer cells in vitro (GI50 (72 h) = 0.32, 0.9, and 1.4 µM in OVCAR-3, OVCAR-8, and NCI/ADR-RES cultures, respectively), and suppress OVCAR-8 tumor growth in mice in vivo via either i.p. (100 mg/kg/5d/wk or 40 mg/kg/d) or p.o. (20 mg/kg/d to 200 mg/kg/d with 20 mg increment every 3 days; then 200 mg/kg/d til day 62). The commonly used PDI inhibitor Bacitracin (Cat. No. 1951) on the other hand is not suitable for in vivo studies due to its nephrotoxicity and low membrane permeability. |
| Catalogue Number | 539225 |
| Brand Family | Calbiochem® |
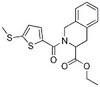
| Synonyms | PDI Inhibitor III, Ethyl 2-(2-(N-(2,4-dimethoxyphenyl)propiolamido)-2-(thiophen-2-yl)acetamido)acetate |
| References | |
|---|---|
| References | Xu, S., et al. 2012. Proc. Natl. Acad. Sci. USA 109, 16348. |
| Product Information | |
|---|---|
| Form | Light beige solid |
| Hill Formula | C₂₁H₂₂N₂O₆S |
| Chemical formula | C₂₁H₂₂N₂O₆S |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PDI |
| Primary Target IC<sub>50</sub> | 10 µ |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 539225-25MG | 04055977268966 |
Documentation
Literatur
| Übersicht |
|---|
| Xu, S., et al. 2012. Proc. Natl. Acad. Sci. USA 109, 16348. |
| Datenblatt | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|