475949 Sigma-AldrichMPS1 Inhibitor, NMS-P715 - CAS 1202055-32-0 - Calbiochem
MPS1 Inhibitor, NMS-P715, CAS 1202055-32-0, is an orally bioavailable, ATP-competitive, reversible, and time-dependent inhibitor of MPS1 (IC50 = 182 nM, Ki = 0.99 nM).
More>> MPS1 Inhibitor, NMS-P715, CAS 1202055-32-0, is an orally bioavailable, ATP-competitive, reversible, and time-dependent inhibitor of MPS1 (IC50 = 182 nM, Ki = 0.99 nM). Less<<Synonyme: (N-(2,6-diethylphenyl)-1-methyl-8-({4-[(1-methylpiperidin-4-yl)carbamoyl]-2-(trifluoromethoxy)phenyl}amino)-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide)
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
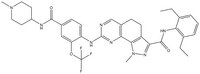
| 1202055-32-0 | C₃₅H₃₉F₃N₈O₃ |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 475949-5MG |
|
Glasflasche | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | An orally bioavailable, ATP-competitive, pyrazolo-quinazoline, MPS1 inhibitor (IC50 = 182 nM, Ki = 0.99 nM) that is shown to act in a reversible and time-dependent manner. It demonstrates selectivity for MPS1 against a panel of 60 kinases, displaying activity against only three kinases, CK2, MELK, and NEK6 (< 10 µM), but not against other mitotic kinases including PLK1, CDK1, Aurora A, Aurora B, or the SAC kinase BUB1, in an in vitro kinase assay. It promotes massive SAC (spindle assembly checkpoint) override (EC50 = 65 nM) in nocodazole-arrested U20S cells and elicits a reduction in the G1 and G2/M phase of the cell cycle in A2780 ovarian cancer cells, similar to RNAi-mediated MPS1 silencing. In addition, it is shown to inactivate SAC, delocalize kinetochore components, and inhibit the proliferation of select cancer cell lines (IC50 ~ 1 µM), without marked activity among a panel of 127 normal cell lines. Also, it inhibits A2780 tumor xenograft growth in mice (90 mg/kg/day, o.s., in vivo) by 53% without marked body weight loss. Also available as a 5 mM solution in DMSO (Cat. No. 506313). |
| Catalogue Number | 475949 |
| Brand Family | Calbiochem® |
| Synonyms | (N-(2,6-diethylphenyl)-1-methyl-8-({4-[(1-methylpiperidin-4-yl)carbamoyl]-2-(trifluoromethoxy)phenyl}amino)-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide) |
| References | |
|---|---|
| References | Caldarelli, M., et al. 2011. Bioorg. Med. Chem. Lett. 21, 4507. Colombo, R., et al. 2011. Cancer Res. 70, 10255. |
| Product Information | |
|---|---|
| CAS number | 1202055-32-0 |
| Form | White powder |
| Hill Formula | C₃₅H₃₉F₃N₈O₃ |
| Chemical formula | C₃₅H₃₉F₃N₈O₃ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | MPS1 Inhibitor, NMS-P715, CAS 1202055-32-0, is an orally bioavailable, ATP-competitive, reversible, and time-dependent inhibitor of MPS1 (IC50 = 182 nM, Ki = 0.99 nM). |
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 475949-5MG | 04055977184273 |
Documentation
MPS1 Inhibitor, NMS-P715 - CAS 1202055-32-0 - Calbiochem SDB
| Titel |
|---|
MPS1 Inhibitor, NMS-P715 - CAS 1202055-32-0 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 475949 |
Literatur
| Übersicht |
|---|
| Caldarelli, M., et al. 2011. Bioorg. Med. Chem. Lett. 21, 4507. Colombo, R., et al. 2011. Cancer Res. 70, 10255. |