444152 Sigma-AldrichMDM2 Antagonist IV, Nutlin-3a - CAS 675576-98-4 - Calbiochem
The MDM2 Antagonist IV, Nutlin-3a, also referenced under CAS 675576-98-4, controls the biological activity of MDM2. This small molecule/inhibitor is primarily used for Cancer applications.
More>> The MDM2 Antagonist IV, Nutlin-3a, also referenced under CAS 675576-98-4, controls the biological activity of MDM2. This small molecule/inhibitor is primarily used for Cancer applications. Less<<Synonyme: (-)-4-(4,5-bis-(4-Chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydroimidazole-1-carbonyl)piperazine-2-one
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
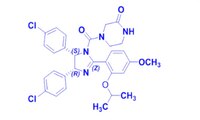
| 675576-98-4 | C₃₀H₃₀Cl₂N₄O₄ |
| Description | |
|---|---|
| Overview | A cell-permeable and highly potent active enantiomer of Nutlin-3 (Cat. No. 444143) that binds to the p53-binding pocket and blocks the interaction of p53 and MDM2 (IC50 = 90 nM). Exhibits over 150-fold greater affinity for MDM2 than its less active enantiomer, Nutlin-3b. Induces p53 mediated apoptosis by both transcription-dependent and independent mechanisms. Shown to greatly potentiate the cytotoxic effects of chemotherapeutic agents and reduce tumor burden in vivo. Also shown to overcome ATM-mediated resistance to fludarabine in chronic lymphocyte leukemia. Cells treated with Nutlin-3a permanently lose their ability to proliferate and enter into a pattern of permanent senescence. Mouse embryonic fibroblasts with p53+/+MEFs show significantly reduced reprogramming capabilities following Nutlin-3a treatment. |
| Catalogue Number | 444152 |
| Brand Family | Calbiochem® |
| Synonyms | (-)-4-(4,5-bis-(4-Chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydroimidazole-1-carbonyl)piperazine-2-one |
| Product Information | |
|---|---|
| CAS number | 675576-98-4 |
| Form | White powder |
| Hill Formula | C₃₀H₃₀Cl₂N₄O₄ |
| Chemical formula | C₃₀H₃₀Cl₂N₄O₄ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 444152 | 0 |
Documentation
MDM2 Antagonist IV, Nutlin-3a - CAS 675576-98-4 - Calbiochem SDB
| Titel |
|---|
MDM2 Antagonist IV, Nutlin-3a - CAS 675576-98-4 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 444152 |
Literatur
| Übersicht |
|---|
| Ohnstad, H.O., et al. 2011. BMC Cancer 11, 211. Shen, H. and Maki, C.G., 2010. J. Biol. Chem. 285, 23105. Kawamura, T., et al. 2009. Nature 460, 1140. Kojima, K., et al. 2006. Blood 108, 993. Laurie, N.A., et al. 2006. Nature 444, 61. Tovar, C., et al. 2006. Proc. Natl. Acad. Sci. USA 103, 1888. Vassilev, L.T. 2006. Trends Mol Med. 13, 23. Thompson, T., et al. 2004. J. Biol. Chem. 279, 53015. Vassilev, L.T., et al. 2004. Science 303, 844. |
| Datenblatt | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|