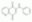440205 Sigma-AldrichLY 83583 - CAS 91300-60-6 - Calbiochem
A cell-permeable, competitive inhibitor of soluble guanylate cyclase (IC₅₀ = 2 µM).
More>> A cell-permeable, competitive inhibitor of soluble guanylate cyclase (IC₅₀ = 2 µM). Less<<Synonyme: 6-Anilino-5,8-quinolinequinone
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 91300-60-6 | C₁₅H₁₀N₂O₂ |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 440205-5MG |
|
Kst.-Ampulle | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 91300-60-6 |
| ATP Competitive | N |
| Form | Reddish purple solid |
| Hill Formula | C₁₅H₁₀N₂O₂ |
| Chemical formula | C₁₅H₁₀N₂O₂ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | soluble guanylate cyclase |
| Primary Target IC<sub>50</sub> | 2 µM against soluble guanylate cyclase |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 440205-5MG | 04055977186727 |
Documentation
LY 83583 - CAS 91300-60-6 - Calbiochem SDB
| Titel |
|---|
LY 83583 - CAS 91300-60-6 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 440205 |
Literatur
| Übersicht |
|---|
| Adachi, R., et al. 2000. Int. J. Immunopharmacol. 22, 855. Kawada, T., et al. 1994. Gen. Pharmacol. 25, 1361. Beasley, D., et al. 1991. J. Clin. Invest. 87, 602. Pandol, S.J., et al. 1990. J. Biol. Chem. 265, 12846. Light, D.B., et al. 1989. Science 243, 383. Mulsch, A. 1988. J. Pharmacol. Exp. Ther. 247, 283. Fleisch, J.H., et al. 1984. J. Pharmacol. Exp. Ther. 229, 681. |
Broschüre
| Titel |
|---|
| Pathways and Biomarkers of Oxidative Stress |