440204 Sigma-AldrichInSolution™ LY 294002 - CAS 154447-36-6 - Calbiochem
InSolution™ LY 294002, CAS 154447-36-6, is a 10 mM solution of LY 294002 (Cat. No. 440202) in DMSO. A cell-permeable, potent, reversible and specific inhibitor of PI 3-kinase (IC50 = 1.4 µM).
More>> InSolution™ LY 294002, CAS 154447-36-6, is a 10 mM solution of LY 294002 (Cat. No. 440202) in DMSO. A cell-permeable, potent, reversible and specific inhibitor of PI 3-kinase (IC50 = 1.4 µM). Less<<Synonyme: 2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one, BRD2 Inhibitor IV, BRD3 Inhibitor III, BRD4 Inhibitor IV
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 154447-36-6 | C₁₉H₁₇NO₃ |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 440204-1MG |
|
Kst.-Ampulle | 1 mg |
|
— |
| Description | |
|---|---|
| Catalogue Number | 440204 |
| Brand Family | Calbiochem® |
| Synonyms | 2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one, BRD2 Inhibitor IV, BRD3 Inhibitor III, BRD4 Inhibitor IV |
| References | |
|---|---|
| References | Dittman, A., et al. 2013. ACS Chem. Biol. 9, 495. Baumann, P., and West, S.C. 1998. Proc. Natl. Acad. Sci. USA 95, 14066. Cardone, M.H., et al. 1998. Science 282, 1318. Vlahos, C.J., et al. 1995. J. Immunol. 154, 2413. Yano, H., et al. 1995. Biochem. J. 312, 145. Vlahos, C.J., et al. 1994. J. Biol. Chem. 269, 5241. Selected Citations Lee, J., et al. 2009. Cell Stem Cell 5, 76. |
| Product Information | |
|---|---|
| CAS number | 154447-36-6 |
| ATP Competitive | Y |
| Form | Liquid |
| Formulation | A 10 mM (1 mg/325 µl) solution of LY 294002 (Cat. No. 440202) in DMSO. |
| Hill Formula | C₁₉H₁₇NO₃ |
| Chemical formula | C₁₉H₁₇NO₃ |
| Reversible | Y |
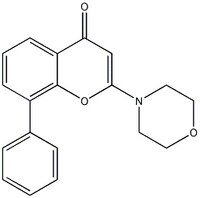
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | phosphatidylinositol 3-kinase |
| Primary Target IC<sub>50</sub> | 1.4 µM against phosphatidylinositol 3-kinase |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 440204-1MG | 04055977186710 |
Documentation
InSolution™ LY 294002 - CAS 154447-36-6 - Calbiochem SDB
| Titel |
|---|
InSolution™ LY 294002 - CAS 154447-36-6 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 440204 |
Literatur
| Übersicht |
|---|
| Dittman, A., et al. 2013. ACS Chem. Biol. 9, 495. Baumann, P., and West, S.C. 1998. Proc. Natl. Acad. Sci. USA 95, 14066. Cardone, M.H., et al. 1998. Science 282, 1318. Vlahos, C.J., et al. 1995. J. Immunol. 154, 2413. Yano, H., et al. 1995. Biochem. J. 312, 145. Vlahos, C.J., et al. 1994. J. Biol. Chem. 269, 5241. Selected Citations Lee, J., et al. 2009. Cell Stem Cell 5, 76. |
Literaturstellen
| Titel | |
|---|---|
|
|
| Datenblatt | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|