217708 Sigma-AldrichCdc42/Rac1 GTPase Inhibitor, ML141 - Calbiochem
Cdc42/Rac1 GTPase Inhibitor, ML141, CAS 71203-35-5, is a cell-permeable, allosteric, potent, selective, reversible, non-competitive inhibitor of Cdc42 GTPases.
More>> Cdc42/Rac1 GTPase Inhibitor, ML141, CAS 71203-35-5, is a cell-permeable, allosteric, potent, selective, reversible, non-competitive inhibitor of Cdc42 GTPases. Less<<Synonyme: CID2950007, (±)-4-(5-(4-Methoxyphenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl))-benzenesulfonamide, (±)-4-(4,5-Dihydro-5-(4-methoxyphenyl)-3-phenyl-1H-pyrazol-1-yl)-benzenesulfonamide, MLS00693334, CDC42 GTPase Inhibitor II, CDC42 Inhibitor II, Rac1 Inhibitor V, Rac1 GTPase Inhibitor II
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 71203-35-5 | C₂₂H₂₁N₃O₃S |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 217708-25MG |
|
Glasflasche | 25 mg |
|
— |
| Description | |
|---|---|
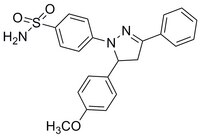
| Overview | A cell-permeable, allosteric, trisubstituted dihydropyrazolyl compound that acts as a potent, selective, reversible and non-competitive inhibitor of Cdc42 GTPases (IC50 = 0.2, 2.6 and 5.4 µM against nucleotide depleted Cdc42-wt, Cdc42-wt and Cdc42 activated mutant, respectively) with excellent selectivity over Rho family GTPases (IC50 > 100 µM for Ras-wt, Ras activated mutant, Rab7-wt, Rab2a-wt, Rac1-wt and Rac1 activated mutant). Shown to efficiently block Cdc42 association with GTPγS and PAK-PBD, and decrease GTP-Cdc42 (> 95%) and GTP-Rac1 (≥40%) contents in EGF-stimulated 3T3 cells, and inhibit Bradykinin (Cat. No. 05-23-0500)-induced filopodia formation in 3T3 cells at 10 µM. |
| Catalogue Number | 217708 |
| Brand Family | Calbiochem® |
| Synonyms | CID2950007, (±)-4-(5-(4-Methoxyphenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl))-benzenesulfonamide, (±)-4-(4,5-Dihydro-5-(4-methoxyphenyl)-3-phenyl-1H-pyrazol-1-yl)-benzenesulfonamide, MLS00693334, CDC42 GTPase Inhibitor II, CDC42 Inhibitor II, Rac1 Inhibitor V, Rac1 GTPase Inhibitor II |
| Product Information | |
|---|---|
| CAS number | 71203-35-5 |
| Form | Yellow-white solid |
| Hill Formula | C₂₂H₂₁N₃O₃S |
| Chemical formula | C₂₂H₂₁N₃O₃S |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | Cdc42/Rac1 GTPase Inhibitor, ML141, CAS 71203-35-5, is a cell-permeable, allosteric, potent, selective, reversible, non-competitive inhibitor of Cdc42 GTPases. |
| Biological Information | |
|---|---|
| Purity | ≥99% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 217708-25MG | 04055977202366 |
Documentation
Cdc42/Rac1 GTPase Inhibitor, ML141 - Calbiochem SDB
| Titel |
|---|
Cdc42/Rac1 GTPase Inhibitor, ML141 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 217708 |
Literatur
| Übersicht |
|---|
| Hong, L., et al. 2012. J. Biol. Chem. in press. Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. 2010 Feb 27. (http://www.ncbi.nlm.nih.gov/books/NBK51965/) |
| Datenblatt | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|