208743 Sigma-AldrichCalpain Inhibitor XI - CAS 145731-49-3 - Calbiochem
The Calpain Inhibitor XI, also referenced under CAS 145731-49-3, controls the biological activity of Calpain. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
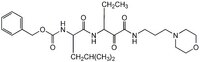
More>> The Calpain Inhibitor XI, also referenced under CAS 145731-49-3, controls the biological activity of Calpain. This small molecule/inhibitor is primarily used for Protease Inhibitors applications. Less<<Synonyme: Z-L-Abu-CONH(CH₂)₃-morpholine
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 145731-49-3 | C₂₆H₄₀N₄O₆ |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 208743-5MG |
|
Kst.-Ampulle | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 145731-49-3 |
| ATP Competitive | N |
| Form | White to off-white solid |
| Formulation | Supplied as a trifluoroacetate salt. |
| Hill Formula | C₂₆H₄₀N₄O₆ |
| Chemical formula | C₂₆H₄₀N₄O₆ |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | calpain 1, calpain 2 |
| Primary Target K<sub>i</sub> | 140 nM and 41 nM, against calpain-1 and -2, respectively |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Peptide Sequence | Z-Leu-Abu-CONH(CH₂)₃-morpholine (Abu = α-aminobutyric acid) |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 208743-5MG | 04055977202809 |
Documentation
Calpain Inhibitor XI - CAS 145731-49-3 - Calbiochem SDB
| Titel |
|---|
Calpain Inhibitor XI - CAS 145731-49-3 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 208743 |
Literatur
| Übersicht |
|---|
| Blomgren, K., et al. 2001. J. Biol. Chem. 276, 10191. DeBiasi, R.L., et al. 2001. J. Virol. 75, 351. Saatman, K.E., et al. 2000. J. Cereb. Blood Flow Metab. 20, 66. Stelmasiak, Z., et al. 2000. Med. Sci. Monit. 6, 426. Blomgren, K., et al. 1999. J. Biol. Chem. 274, 14046. James, T., et al. 1998. J. Neurosci. Res. 51, 218. Li, Z., et al. 1996. J. Med. Chem. 39, 4089. Saatman, K.E, et al. 1996. Proc. Natl. Acad. Sci. USA 93, 3428. Bartus, R.T., et al. 1995. Neurol. Res. 17, 249. Bartus, R.T., et al. 1994. Stroke 25, 2265. |