189291 Sigma-AldrichAtorvastatin, Calcium Salt - CAS 134523-03-8 - Calbiochem
Atorvastatin, Calcium Salt, CAS 134523-03-8, is a cell-permeable, highly potent, and competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (IC50 = 7.5 nM in rat liver).
More>> Atorvastatin, Calcium Salt, CAS 134523-03-8, is a cell-permeable, highly potent, and competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (IC50 = 7.5 nM in rat liver). Less<<Synonyme: (3R,5R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate, hemicalcium salt,
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
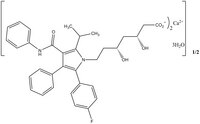
| 134523-03-8 | [(C₃₃H₃₄FN₂O₅)2Ca•3H₂O]1/2 |
| Product Information | |
|---|---|
| CAS number | 134523-03-8 |
| Form | White powder |
| Hill Formula | [(C₃₃H₃₄FN₂O₅)2Ca•3H₂O]1/2 |
| Chemical formula | [(C₃₃H₃₄FN₂O₅)2Ca•3H₂O]1/2 |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 189291 | 0 |
Documentation
Atorvastatin, Calcium Salt - CAS 134523-03-8 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 189291 |
Literatur
| Übersicht |
|---|
| Chen, J., et al. 2012. Int. J. Biochem. Cell Biol., in press. Tycinska, A.M., et al. 2011. Arch. Med. Sci. 7, 955. Law, M.R., et al. 2003. BMJ 326, 1423. Youssef, S., et al. 2002. Nature 420, 78. Burnett, J.R., et al. 1998. Arterioscler. Thromb. Vasc. Biol. 18, 1906. Bustos, C., et al. 1998. J. Am. Coll. Cardiol. 32, 2057. Bocan, T.M.A., et al. 1992. Biochimica et Biophysica Acta 1123, 133. Roth, B.D., et al. 1991. J. Med. Chem. 34, 463. Shaw, M.K., et al. 1990. Biochem. Biophys. Res. Commun. 170, 726. |