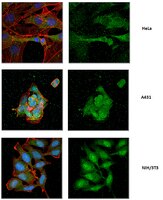
Exo70 generates membrane curvature for morphogenesis and cell migration.
Zhao, Yuting, et al.
Dev. Cell, 26: 266-78 (2013)
2013
Abstract anzeigen
Dynamic shape changes of the plasma membrane are fundamental to many processes, ranging from morphogenesis and cell migration to phagocytosis and viral propagation. Here, we demonstrate that Exo70, a component of the exocyst complex, induces tubular membrane invaginations toward the lumen of synthetic vesicles in vitro and generates protrusions on the surface of cells. Biochemical analyses using Exo70 mutants and independent molecular dynamics simulations based on Exo70 structure demonstrate that Exo70 generates negative membrane curvature through an oligomerization-based mechanism. In cells, the membrane-deformation function of Exo70 is required for protrusion formation and directional cell migration. Exo70 thus represents a membrane-bending protein that may couple actin dynamics and plasma membrane remodeling for morphogenesis. | 23948253
 |
Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells.
Liu, Jianglan, et al.
Mol. Biol. Cell, 18: 4483-92 (2007)
2007
Abstract anzeigen
The exocyst is an evolutionarily conserved octameric protein complex that tethers post-Golgi secretory vesicles at the plasma membrane for exocytosis. To elucidate the mechanism of vesicle tethering, it is important to understand how the exocyst physically associates with the plasma membrane (PM). In this study, we report that the mammalian exocyst subunit Exo70 associates with the PM through its direct interaction with phosphatidylinositol 4,5-bisphosphate (PI(4,5)P(2)). Furthermore, we have identified key conserved residues at the C-terminus of Exo70 that are crucial for the interaction of Exo70 with PI(4,5)P(2). Disrupting Exo70-PI(4,5)P(2) interaction abolished the membrane association of Exo70. We have also found that wild-type Exo70 but not the PI(4,5)P(2)-binding-deficient Exo70 mutant is capable of recruiting other exocyst components to the PM. Using the ts045 vesicular stomatitis virus glycoprotein trafficking assay, we demonstrate that Exo70-PI(4,5)P(2) interaction is critical for the docking and fusion of post-Golgi secretory vesicles, but not for their transport to the PM. | 17761530
 |