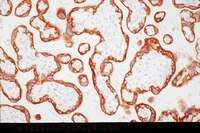
M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia.
Smith, AM; Gibbons, HM; Oldfield, RL; Bergin, PM; Mee, EW; Curtis, MA; Faull, RL; Dragunow, M
Journal of neuroinflammation
10
85
2013
Abstract anzeigen
Microglia are the primary immune cells of the brain whose phenotype largely depends on their surrounding micro-environment. Microglia respond to a multitude of soluble molecules produced by a variety of brain cells. Macrophage colony-stimulating factor (M-CSF) is a cytokine found in the brain whose receptor is expressed by microglia. Previous studies suggest a critical role for M-CSF in brain development and normal functioning as well as in several disease processes involving neuroinflammation.Using biopsy tissue from patients with intractable temporal epilepsy and autopsy tissue, we cultured primary adult human microglia to investigate their response to M-CSF. Mixed glial cultures were treated with 25 ng/ml M-CSF for 96 hours. Proliferation and phagocytosis assays, and high through-put immunocytochemistry, microscopy and image analysis were performed to investigate microglial phenotype and function.We found that the phenotype of primary adult human microglia was markedly changed following exposure to M-CSF. A greater number of microglia were present in the M-CSF- treated cultures as the percentage of proliferating (BrdU and Ki67-positive) microglia was greatly increased. A number of changes in protein expression occurred following M-CSF treatment, including increased transcription factors PU.1 and C/EBPβ, increased DAP12 adaptor protein, increased M-CSF receptor (CSF-1R) and IGF-1 receptor, and reduced HLA-DP, DQ, DR antigen presentation protein. Furthermore, a distinct morphological change was observed with elongation of microglial processes. These changes in phenotype were accompanied by a functional increase in phagocytosis of Aβ1-42 peptide.We show here that the cytokine M-CSF dramatically influences the phenotype of adult human microglia. These results pave the way for future investigation of M-CSF-related targets for human therapeutic benefit. | | 23866312
 |
Insulin-like growth factor-I E-peptide activity is dependent on the IGF-I receptor.
Brisson, BK; Barton, ER
PloS one
7
e45588
2011
Abstract anzeigen
Insulin-like growth factor-I (IGF-I) is an essential growth factor that regulates the processes necessary for cell proliferation, differentiation, and survival. The Igf1 gene encodes mature IGF-I and a carboxy-terminal extension called the E-peptide. In rodents, alternative splicing and post-translational processing produce two E-peptides (EA and EB). EB has been studied extensively and has been reported to promote cell proliferation and migration independently of IGF-I and its receptor (IGF-IR), but the mechanism by which EB causes these actions has not been identified. Further, the properties of EA have not been evaluated. Therefore, the goals of this study were to determine if EA and EB possessed similar activity and if these actions were IGF-IR independent. We utilized synthetic peptides for EA, EB, and a scrambled control to examine cellular responses. Both E-peptides increased MAPK signaling, which was blocked by pharmacologic IGF-IR inhibition. Although the E-peptides did not directly induce IGF-IR phosphorylation, the presence of either E-peptide increased IGF-IR activation by IGF-I, and this was achieved through enhanced cell surface bioavailability of the receptor. To determine if E-peptide biological actions required the IGF-IR, we took advantage of the murine C2C12 cell line as a platform to examine the key steps of skeletal muscle proliferation, migration and differentiation. EB increased myoblast proliferation and migration while EA delayed differentiation. The proliferation and migration effects were inhibited by MAPK or IGF-IR signaling blockade. Thus, in contrast to previous studies, we find that E-peptide signaling, mitogenic, and motogenic effects are dependent upon IGF-IR. We propose that the E-peptides have little independent activity, but instead affect growth via modulating IGF-I signaling, thereby increasing the complexity of IGF-I biological activity. | | 23029120
 |
Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group.
Malempati, S; Weigel, B; Ingle, AM; Ahern, CH; Carroll, JM; Roberts, CT; Reid, JM; Schmechel, S; Voss, SD; Cho, SY; Chen, HX; Krailo, MD; Adamson, PC; Blaney, SM
Journal of clinical oncology : official journal of the American Society of Clinical Oncology
30
256-62
2011
Abstract anzeigen
A phase I/II study of cixutumumab (IMC-A12) in children with refractory solid tumors was conducted. This study was designed to assess the toxicities, pharmacokinetics, and pharmacodynamics of cixutumumab in children to determine a recommended phase II dose and to assess antitumor activity in Ewing sarcoma (ES).Pediatric patients with relapsed or refractory solid tumors were treated with cixutumumab as a 1-hour intravenous infusion once per week. Two dose levels-6 and 9 mg/kg-were evaluated using a standard three-plus-three cohort design. Patients with refractory ES were treated in an expanded phase II cohort at each dose level.Forty-seven eligible patients with a median age of 15 years (range, 4 to 28 years) were enrolled. Twelve patients were treated in the dose-finding phase. Hematologic and nonhematologic toxicities were generally mild and infrequent. Dose-limiting toxicities included grade 4 thrombocytopenia at 6 mg/kg and grade 3 dehydration at 9 mg/kg. Mean trough concentration (± standard deviation) at 9 mg/kg was 106 ± 57 μg/mL, which exceeded the effective trough concentration of 60 μg/mL observed in xenograft models. Three patients with ES had confirmed partial responses: one of 10 at 6 mg/kg and two of 20 at 9 mg/kg. Serum insulin-like growth factor I (IGF-I) levels consistently increased after one dose of cixutumumab. Tumor IGF-I receptor expression by immunohistochemistry did not correlate with response in patients with ES.Cixutumumab is well tolerated in children with refractory solid tumors. The recommended phase II dose is 9 mg/kg. Limited single-agent activity of cixutumumab was seen in ES. | Immunohistochemistry (Paraffin) | 22184397
 |
Epidermal growth factor receptor (EGFR) is overexpressed in high-grade dysplasia and adenocarcinoma of the esophagus and may represent a biomarker of histological progression in Barrett's esophagus (BE).
Cronin, J; McAdam, E; Danikas, A; Tselepis, C; Griffiths, P; Baxter, J; Thomas, L; Manson, J; Jenkins, G
The American journal of gastroenterology
106
46-56
2010
Abstract anzeigen
The assessment of cancer risk in patients with Barrett's esophagus (BE) is currently fraught with difficulty. The current gold standard method of assessing cancer risk is histological assessment, with the appearance of high-grade dysplasia (HGD) as the key event monitored. Sampling error during endoscopy limits the usefulness of this approach, and there has been much recent interest in supplementing histological assessment with molecular markers, which may aid in patient stratification.No molecular marker has been yet validated to accurately correlate with esophageal histological progression. Here, we assessed the suitability of several membranous proteins as biomarkers by correlating their abundance with histological progression. In all, 107 patient samples, from 100 patients, were arranged on a tissue microarray (TMA) and represented the various stages of histological progression in BE. This TMA was probed with antibodies for eight receptor proteins (mostly membranous).Epidermal growth factor receptor (EGFR) staining was found to be the most promising biomarker identified with clear increases in staining accompanying histological progression. Further, immunohistochemistry was performed using the full-tissue sections from BE, HGD, and adenocarcinoma tissues, which confirmed the stepwise increase in EGFR abundance. Using a robust H-score analysis, EGFR abundance was shown to increase 13-fold in the adenocarcinoma tissues compared to the BE tissues. EGFR was "overexpressed" in 35% of HGD specimens and 80% of adenocarcinoma specimens when using the H-score of the BE patients (plus 3 s.d.) as the threshold to define overexpression. EGFR staining was also noted to be higher in BE tissues adjacent to HGD/adenocarcinoma. Western blotting, although showing more EGFR protein in the adenocarcinomas compared to the BE tissue, was highly variable. EGFR overexpression was accompanied by aneuploidy (gain) of chromosome 7, plus amplification of the EGFR locus. Finally, the bile acid deoxycholic acid (DCA) (at neutral and acidic pH) and acid alone was capable of upregulating EGFR mRNA in vitro, and in the case of neutral pH DCA, this was NF-κB dependent.EGFR is overexpressed during the histological progression in BE tissues and hence may be useful as a biomarker of histological progression. Furthermore, as EGFR is a membranous protein expressed on the luminal surface of the esophageal mucosa, it may also be a useful target for biopsy guidance during endoscopy. | | 21157443
 |
Role of IGFs and insulin in the human testis during postnatal activation: differentiation of steroidogenic cells.
Berensztein, EB; Baquedano, MS; Pepe, CM; Costanzo, M; Saraco, NI; Ponzio, R; Rivarola, MA; Belgorosky, A
Pediatric research
63
662-6
2008
Abstract anzeigen
Immunoexpression of IGF-I, IGF-II, type 1 IGF receptor (IGFR), insulin receptor (IR), and GH receptor (GHR) was analyzed in human testis, in three age groups (Gr): Gr1 (neonates), Gr2 (postnatal testicular activation), and Gr3 (early prepuberty). In interstitial cells, low IGF-I and GHR, but moderate IR immunoexpression was observed in all Grs. However, high expression of IGF-II in Gr1, and moderate expression of IGFR in Gr1 and Gr2 were found. In Leydig cell (LC), high expression of IGF-II, moderate expression of IGFR and GHR, and undetectable IGF-I was found. Moreover, IR was highly expressed in Gr2. The effect of IGF-I on cell proliferation (PI) and apoptosis (AI), induction of cytochrome P450 side chain cleavage (cP450scc) immunoexpression, 3beta-hydroxysteroid dehydrogenase mRNA and testosterone (T) secretion was evaluated in human testis cell cultures. IGF-I increased P450scc immunoexpression, 3beta-hydroxysteroid dehydrogenase mRNA, T secretion, and PI, but decreased AI. We propose that IGF-II, mainly through IR, is involved in functional LC differentiation. In some interstitial cells, probably in LC precursors, IGF-II/IR could be involved, among other factors, in the stimulation of PI and/or inhibition of AI, and in LC differentiation. | | 18520331
 |
Clinical implications of insulin-like growth factor 1 system in early-stage cervical cancer.
Huang, YF; Shen, MR; Hsu, KF; Cheng, YM; Chou, CY
British journal of cancer
99
1096-102
2008
Abstract anzeigen
This study was aimed to identify the expression and the correlation of insulin-like growth factor-1 (IGF-1) system and their prognostic impacts in cervical cancer. Seventy-two patients with early-stage cervical cancer were eligible. We obtained the serum levels of total IGF-1 and IGF binding protein-3 (IGFBP-3) by enzyme-linked immunosorbent assay and the expression of IGF-1 receptor (IGF-1R) in cancerous tissue by immuno-fluorescent (IF) stains. The 5-year recurrence-free and overall survival rates were significantly lower (P=0.003 and P=0.01, respectively) among patients with high-grade expression of tissue IGF-1R, compared with those with low-grade expression. After adjustment for other factors, preoperative serum total IGF-1 or IGFBP-3 levels failed to predict cancer death and recurrence. High-grade expression of IGF-1R and elevated preoperative squamous cell carcinoma antigen level were independent predictors of both death and recurrence, and combination of both factors could further help identify the subgroup of patients at higher death risk. The IF staining indicates the colocalisation of IGF-1 and IGF-1R in the cancerous tissues, whereas the IGF-1R expression is not correlated with circulating levels of IGF-1 or IGFBP-3. In early-stage cervical cancer, IGF-1 system may have a paracrine or autocrine function and the adverse impacts on prognosis by IGF-1R overexpression are implicated. | | 18781172
 |
Expression of the IGF system in human adrenal tissues from early infancy to late puberty: implications for the development of adrenarche.
Baquedano, MS; Berensztein, E; Saraco, N; Dorn, GV; de Davila, MT; Rivarola, MA; Belgorosky, A
Pediatric research
58
451-8
2004
Abstract anzeigen
IGF-1, IGF-2, and type 1 IGF receptor (IGF-R1) mRNA expression and immunolocalization and cell proliferation index were studied in human adrenals from early infancy to late puberty. Adrenals were obtained from transplantation donors or from necropsies of endocrinologically normal subjects. Subjects were divided into three age groups: group 1, less than 3 mo of age, involution of fetal adrenals; group 2, 3 mo to 6 y of age, preadrenarche; and group 3, older than 6 y up to 20 y of age, postadrenarche. Cell proliferation index (Ki-67) in the outer, subcapsular, zona glomerulosa was significantly higher than in zona fasciculata of all groups and in zona reticularis or fetal zone. IGF-1 mRNA (semiquantitative reverse transcriptase-PCR and Northern blot) in group 2 was significantly higher than in group 1 and group 3 (p less than 0.05). IGF2 mRNA in group 1 was significantly higher than in the other groups. IGF-R1 mRNA in group 3 was significantly higher than in group 2 but not different from group 1. Strong IGF-1, IGF-2, and IGF-R1 immunostaining signal was observed in the outer, subcapsular, zona glomerulosa and in zona fasciculata in the three groups, whereas a very weak IGF-1 and IGF-R1 immunostaining signal was found in fetal zone cells of group 1 and in zona reticularis of group 3. We propose that IGF-1 could be a factor involved in the postnatal mechanism of progenitor adrenal cell proliferation and migration. Our data also suggest that IGF-1 is not a direct regulatory factor of adrenal androgen production by zona reticularis cells. | | 16148056
 |
Low levels of insulin-like growth factor type 1 receptor expression at cancer cell membrane predict liver metastasis in Dukes' C human colorectal cancers.
Nakamura, M; Miyamoto, S; Maeda, H; Zhang, SC; Sangai, T; Ishii, G; Hasebe, T; Endoh, Y; Saito, N; Asaka, M; Ochiai, A
Clinical cancer research : an official journal of the American Association for Cancer Research
10
8434-41
2004
Abstract anzeigen
The aim of this study was to evaluate the prognostic significance of insulin-like growth factor type 1 receptor (IGF-1R) expression in Dukes' C human colorectal cancers (CRCs).Immunohistochemical staining for IGF-1R was done on formalin-fixed, paraffin-embedded specimens from 161 patients with curatively resected Dukes' C CRC and at least 5-year follow-up periods. We investigated the association between the levels of IGF-1R expression and the clinicopathologic parameters. To evaluate the accurate prognostic value of IGF-1R expression, we investigated two patterns of recurrence-free survival (RFS) according to the mode of recurrence, the hepatic-RFS (H-RFS), and the nonhepatic-RFS (nH-RFS). The influence of the pattern of IGF-1R immunostaining (membranous or cytoplasmic) on RFS was also estimated.High (diffuse staining) and low (focal staining) levels of IGF-1R expression were found in 45 (28%) and 116 (72%) specimens, respectively. The recurrence rate was significantly higher in the latter group (49 of 116) than the former group (9 of 45; P = 0.01). H-RFS was significantly longer for the former group than the latter group (P = 0.021), whereas no difference was found in nH-RFS between the two groups (P = 0.121). In multivariate analysis, the level of IGF-1R expression was an independent factor for H-RFS (P = 0.015) as were the depth of invasion and lymph vessel invasion (P = 0.006 and 0.022, respectively). Using a combination of the level of IGF-1R expression and these two factors, the prognostic value was further increased. When IGF-1R staining patterns (membranous or cytoplasmic) were compared, membrane staining of IGF-1R possessed prognostic significance.In Dukes' C CRC, focal membrane expression of IGF-1R in the primary tumor can predict a high risk of recurrence, especially liver metastasis. Understanding the mechanisms involved could lead to new therapeutic approaches for advanced CRC. | | 15623623
 |
Evidence for mutual interdependence of epithelium and stromal lymphoid cells in a subset of papillary carcinomas.
Takahashi, M H, et al.
Br. J. Cancer, 72: 813-7 (1995)
1994
Abstract anzeigen
We have correlated the morphological features of 30 human thyroid carcinomas with the cellular localisation of insulin-like growth factor 1 (IGF-1) mRNA and IGF-1 receptor peptide using in situ hybridisation with digoxigenin-labelled oligoprobes and immunohistochemistry. Four of the five follicular carcinomas studied showed a consistent, uniform, strong positivity for IGF-1 mRNA in tumour cells compared with weakly positive surrounding normal follicular tissue and negative stroma. The majority of papillary carcinomas showed weak to moderate epithelial positivity for IGF-1 mRNA and negative stroma. Immunohistochemistry for IGF-1 receptor showed moderate positivity confined to the tumour epithelial cells in both follicular and the majority of papillary carcinomas. However, in a subgroup of papillary carcinomas characterised by a diffuse stromal lymphoid infiltration (n = 5), the stromal cells showed a much stronger reactivity for IGF-1 mRNA than the tumour or background thyroid, and the tumour cells showed a uniformly high level of immunoreactivity for IGF-1 receptor. These results are compatible with the growth of the papillary carcinoma in these cases being the result of a symbiotic relationship between the stromal lymphoid cells and the tumour epithelium with the lymphoid cells responding to an antigen produced by the tumour cells and the tumour cells responding to a growth factor produced by the lymphoid infiltrate. We suggest that this mechanism may be important in other tumours regularly associated with a widespread lymphoid infiltrate. | | 7547225
 |
Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity.
Soos, M A, et al.
Biochem. J., 290 ( Pt 2): 419-26 (1993)
1992
Abstract anzeigen
Hybrid insulin/insulin-like growth factor-I (IGF-I) receptors have previously been described in human placenta, but it has not been possible to study their properties in the presence of classical insulin receptors and type I IGF receptors. To facilitate the purification of hybrids, we produced an anti-peptide monoclonal antibody IGFR 1-2, directed against the C-terminal peptide of the type I IGF receptor beta-subunit. The antibody bound native human and rat type I IGF receptors, and reacted specifically with the beta-subunit on immunoblots. Solubilized placental microsomal membranes were depleted of classical type I IGF receptors by incubation with an immobilized monoclonal antibody IGFR 24-55, which reacts well with type I receptors but very poorly with hybrid receptors. Residual hybrid receptors were then isolated by incubation with immobilized antibody IGFR 1-2, and recovered by elution with excess of synthetic peptide antigen. Binding properties of hybrids were compared with those of immuno-affinity-purified insulin receptors and type I IGF receptors, by using the radioligands 125I-IGF-I and 125I-insulin. Hybrids bound approx. 20 times as much 125I-IGF-I as 125I-insulin at tracer concentrations (approx. 0.1 nM). The binding of 125I-insulin, but not 125I-IGF-I, to hybrids increased after treatment with dithiothreitol to reduce disulphide bonds between the alpha-subunits. Hybrids behaved very similarly to type I receptors with respect to the inhibition of 125I-IGF-I binding by unlabelled IGF-I and insulin. By contrast, the affinity of hybrids for insulin was approx. 10-fold lower than that of classical insulin receptors, as assessed by inhibition of 125I-insulin binding by unlabelled hormone. It is concluded that the properties of insulin receptors, but not IGF receptors, are markedly affected by assembly as hybrid compared with classical structures, and that hybrids are more likely to be responsive to IGF-I than insulin under physiological conditions. | | 8452530
 |