Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung.
Sureshbabu, A; Syed, MA; Boddupalli, CS; Dhodapkar, MV; Homer, RJ; Minoo, P; Bhandari, V
Respiratory research
16
4
2015
Abstract anzeigen
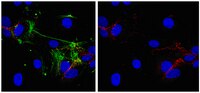
Earlier studies have reported that transforming growth factor beta 1(TGFβ1) is a critical mediator of hyperoxia-induced acute lung injury (HALI) in developing lungs, leading to impaired alveolarization and a pulmonary phenotype of bronchopulmonary dysplasia (BPD). However, the mechanisms responsible for the TGFβ1-induced inflammatory signals that lead to cell death and abnormal alveolarization are poorly understood. We hypothesized that TGFβ1 signaling via TGFβR2 is necessary for the pathogenesis of the BPD pulmonary phenotype resulting from HALI.We utilized lung epithelial cell-specific TGFβ1 overexpressing transgenic and TGFβR2 null mutant mice to evaluate the effects on neonatal mortality as well as pulmonary inflammation and apoptosis in developing lungs. Lung morphometry was performed to determine the impaired alveolarization and multicolor flow cytometry studies were performed to detect inflammatory macrophages and monocytes in lungs. Apoptotic cell death was measured with TUNEL assay, immunohistochemistry and western blotting and protein expression of angiogenic mediators were also analyzed.Our data reveals that increased TGFβ1 expression in newborn mice lungs leads to increased mortality, macrophage and immature monocyte infiltration, apoptotic cell death specifically in Type II alveolar epithelial cells (AECs), impaired alveolarization, and dysregulated angiogenic molecular markers.Our study has demonstrated the potential role of inhibition of TGFβ1 signaling via TGFβR2 for improved survival, reduced inflammation and apoptosis that may provide insights for the development of potential therapeutic strategies targeted against HALI and BPD. | Immunohistochemistry | | 25591994
 |
Multipotent neural crest stem cell-like cells from rat vibrissa dermal papilla induce neuronal differentiation of PC12 cells.
Li, M; Liu, JY; Wang, S; Xu, H; Cui, L; Lv, S; Xu, J; Liu, S; Chi, G; Li, Y
BioMed research international
2014
186239
2014
Abstract anzeigen
Bone marrow mesenchymal stem cells (BMSCs) transplants have been approved for treating central nervous system (CNS) injuries and diseases; however, their clinical applications are limited. Here, we model the therapeutic potential of dermal papilla cells (DPCs) in vitro. DPCs were isolated from rat vibrissae and characterized by immunocytofluorescence, RT-PCR, and multidifferentiation assays. We examined whether these cells could secrete neurotrophic factors (NTFs) by using cocultures of rat pheochromocytoma cells (PC12) with conditioned medium and ELISA assay. DPCs expressed Sox10, P75, Nestin, Sox9, and differentiated into adipocytes, osteoblasts, smooth muscle cells, and neurons under specific inducing conditions. The DPC-conditioned medium (DPC-CM) induced neuronal differentiation of PC12 cells and promoted neurite outgrowth. Results of ELISA assay showed that compared to BMSCs, DPCs secreted more brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF). Moreover, we observed that, compared with the total DPC population, sphere-forming DPCs expressed higher levels of Nestin and P75 and secreted greater amounts of GDNF. The DPCs from craniofacial hair follicle papilla may be a new and promising source for treating CNS injuries and diseases. | Immunocytochemistry | Rat | 25045659
 |
Snail1 controls TGF-β responsiveness and differentiation of mesenchymal stem cells.
Batlle, R, et al.
Oncogene, (2012)
2011
Abstract anzeigen
The Snail1 transcriptional repressor plays a key role in triggering epithelial-to-mesenchymal transition. Although Snail1 is widely expressed in early development, in adult animals it is limited to a subset of mesenchymal cells where it has a largely unknown function. Using a mouse model with inducible depletion of Snail1, here we demonstrate that Snail1 is required to maintain mesenchymal stem cells (MSCs). This effect is associated to the responsiveness to transforming growth factor (TGF)-β1 that shows a strong Snail1 dependence. Snail1 depletion in conditional knockout adult animals causes a significant decrease in the number of bone marrow-derived MSCs. In culture, Snail1-deficient MSCs prematurely differentiate to osteoblasts or adipocytes and, in contrast to controls, are resistant to the TGF-β1-induced differentiation block. These results demonstrate a new role for Snail1 in TGF-β response and MSC maintenance.Oncogene advance online publication, 6 August 2012; doi:10.1038/onc.2012.342. | | | 22869142
 |
















 Antibody[213896-ALL].jpg)