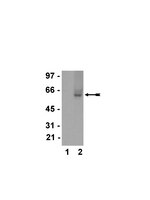
Isolation and characterization of the herpes simplex virus 1 terminase complex.
Heming, JD; Huffman, JB; Jones, LM; Homa, FL
Journal of virology
88
225-36
2014
Abstract anzeigen
During herpes simplex virus 1 (HSV-1) infection, empty procapsids are assembled and subsequently filled with the viral genome by means of a protein complex called the terminase, which is comprised of the HSV-1 UL15, UL28, and UL33 proteins. Biochemical studies of the terminase proteins have been hampered by the inability to purify the intact terminase complex. In this study, terminase complexes were isolated by tandem-affinity purification (TAP) using recombinant viruses expressing either a full-length NTAP-UL28 fusion protein (vFH476) or a C-terminally truncated NTAP-UL28 fusion protein (vFH499). TAP of the UL28 protein from vFH476-infected cells, followed by silver staining, Western blotting, and mass spectrometry, identified the UL15, UL28, and UL33 subunits, while TAP of vFH499-infected cells confirmed previous findings that the C terminus of UL28 is required for UL28 interaction with UL33 and UL15. Analysis of the oligomeric state of the purified complexes by sucrose density gradient ultracentrifugation revealed that the three proteins formed a complex with a molecular mass that is consistent with the formation of a UL15-UL28-UL33 heterotrimer. In order to assess the importance of conserved regions of the UL15 and UL28 proteins, recombinant NTAP-UL28 viruses with mutations of the putative UL28 metal-binding domain or within the UL15 nuclease domain were generated. TAP of UL28 complexes from cells infected with each domain mutant demonstrated that the conserved cysteine residues of the putative UL28 metal-binding domain and conserved amino acids within the UL15 nuclease domain are required for the cleavage and packaging functions of the viral terminase, but not for terminase complex assembly. | Western Blotting | 24155374
 |
The herpes simplex virus 1 UL17 protein is the second constituent of the capsid vertex-specific component required for DNA packaging and retention.
Toropova, K; Huffman, JB; Homa, FL; Conway, JF
Journal of virology
85
7513-22
2010
Abstract anzeigen
The herpes simplex virus (HSV) UL17 and UL25 minor capsid proteins are essential for DNA packaging. They are thought to comprise a molecule arrayed in five copies around each of the capsid vertices. This molecule was initially termed the "C-capsid-specific component" (CCSC) (B. L. Trus et al., Mol. Cell 26:479-489, 2007), but as we have subsequently observed this feature on reconstructions of A, B, and C capsids, we now refer to it more generally as the "capsid vertex-specific component" (CVSC) (S. K. Cockrell et al., J. Virol. 85:4875-4887, 2011). We previously confirmed that UL25 occupies the vertex-distal region of the CVSC density by visualizing a large UL25-specific tag in reconstructions calculated from cryo-electron microscopy (cryo-EM) images. We have pursued the same strategy to determine the capsid location of the UL17 protein. Recombinant viruses were generated that contained either a small tandem affinity purification (TAP) tag or the green fluorescent protein (GFP) attached to the C terminus of UL17. Purification of the TAP-tagged UL17 or a similarly TAP-tagged UL25 protein clearly demonstrated that the two proteins interact. A cryo-EM reconstruction of capsids containing the UL17-GFP protein reveals that UL17 is the second component of the CVSC and suggests that UL17 interfaces with the other CVSC component, UL25, through its C terminus. The portion of UL17 nearest the vertex appears to be poorly constrained, which may provide flexibility in interacting with tegument proteins or the DNA-packaging machinery at the portal vertex. The exposed locations of the UL17 and UL25 proteins on the HSV-1 capsid exterior suggest that they may be attractive targets for highly specific antivirals. | Western Blotting | 21632758
 |
A new expression vector for high level protein production, one step purification and direct isotopic labeling of calmodulin-binding peptide fusion proteins
Zheng, C F, et al
Gene, 186:55-60 (1997)
1997
| | 9047344
 |
Modular binding domains in signal transduction proteins.
Cohen, G B, et al.
Cell, 80: 237-48 (1995)
1994
| | 7834743
 |
Strategies for the identification of interacting proteins.
Guarente, L
Proc. Natl. Acad. Sci. U.S.A., 90: 1639-41 (1993)
1992
Abstract anzeigen
Many problems in modern biology involve complex arrays of interacting protein and, in some cases, RNA molecules. The initial challenge facing investigators is to identify the important players that drive the process under study. This difficult task is ameliorated somewhat by the development of methods designed to keep pace with the magnitude of this challenge. I have outlined a few of these approaches at the cutting edge of cloning interacting proteins. A perhaps more daunting prospect is to dissect the important molecules once they are in hand, to identify key interactions, and, ultimately, to move to an understanding of function in cells. For this, of course, all of the tools of genetics, biochemistry, and molecular biology, extant and yet to be developed, will have to be tapped. | | 8446576
 |
Mammalian Ras interacts directly with the serine/threonine kinase Raf.
Vojtek, A B, et al.
Cell, 74: 205-14 (1993)
1992
Abstract anzeigen
We have identified proteins that interact with H-Ras using a two hybrid system screen of a mouse cDNA library. Approximately 50% of the clones identified encoded portions of the c-Raf and A-Raf serine/threonine kinases. Overlaps among these clones define a conserved 81 residue region of the N-terminus of Raf as the Ras interaction region. We show that Raf interacts with wild-type and activated Ras, but not with an effector domain mutant of Ras or with a dominant-interfering Ras mutant. Using purified bacterially expressed fusion proteins, we show, furthermore, that Ras and the N-terminal region of Raf associate directly in vitro and that this interaction is dependent on GTP bound to Ras. | | 8334704
 |
Affinity purification of histidine-tagged proteins.
Schmitt, J, et al.
Mol. Biol. Rep., 18: 223-30 (1993)
1992
Abstract anzeigen
Expression of recombinant proteins is a standard technique in molecular biology and a wide variety of prokaryotic as well as eukaryotic expression systems are currently in use. A limiting step is often the purification of the expressed recombinant protein, particularly if mammalian expression systems that yield low expression levels are employed. Here, we discuss the advantages and restrictions of tagging recombinant proteins with histidines and purifying them by Ni(2+)-NTA chromatography. | | 8114690
 |
A single step purification for recombinant proteins. Characterization of a microtubule associated protein (MAP 2) fragment which associates with the type II cAMP-dependent protein kinase.
Stofko-Hahn, R E, et al.
FEBS Lett., 302: 274-8 (1992)
1992
Abstract anzeigen
A 167 base pair DNA cassette has been constructed to facilitate the detection and purification of recombinant proteins. This cassette, kfc, encodes three distinct peptide units: a phosphorylation site for the cAMP-dependent protein kinase (PKA), called kemptide, a factor Xa cleavage site, and a calmodulin-binding peptide. Expressed kfc fusion proteins can be purified from bacterial lysates in one step by affinity chromatography on calmodulin-agarose using EGTA as eluant. As a test of this system, we describe the expression, purification and characterization of the PKA binding domain of the microtubule associated protein (MAP 2). | | 1318232
 |
Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif.
Carr, D W, et al.
J. Biol. Chem., 266: 14188-92 (1991)
1991
Abstract anzeigen
The type II cAMP-dependent protein kinase is localized to specific subcellular environments through the binding of the regulatory subunit (RII) dimer to RII-anchoring proteins. Computer-aided analysis of secondary structure, performed on four RII-anchoring protein sequences (the microtubule-associated protein 2, P150, and two thyroid proteins Ht 21 and Ht 31), has identified common regions of approximately 14 residues which display high probabilities of forming amphipathic helices. The potential amphipathic helix region of Ht 31 (Leu-Ile-Glu-Glu-Ala-Ala-Ser-Arg-Ile-Val-Asp-Ala-Val-Ile) lies between residues 494 and 507. A bacterially expressed 318-amino acid fragment, Ht 31 (418-736), containing the amphipathic helix region, was able to bind RII alpha. Site-directed mutagenesis designed to disrupt the secondary structure in the putative binding helix reduced binding dramatically. Specifically, substitution of proline for Ala-498 significantly diminished RII alpha binding, and similar mutation of Ile-502 or Ile-507 abolished interaction. Mutation of Ala-522 to proline, which is located outside the predicted amphipathic helix region, had no effect on RII alpha binding. These data suggest that anchoring proteins interact with RII alpha via an amphipathic helix binding motif. | | 1860836
 |
Use of T7 RNA polymerase to direct expression of cloned genes.
Studier, F W, et al.
Meth. Enzymol., 185: 60-89 (1990)
1990
| | 2199796
 |