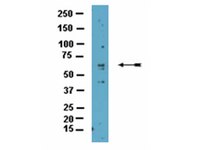
PADI4 acts as a coactivator of Tal1 by counteracting repressive histone arginine methylation.
Kolodziej, S; Kuvardina, ON; Oellerich, T; Herglotz, J; Backert, I; Kohrs, N; Buscató, El; Wittmann, SK; Salinas-Riester, G; Bonig, H; Karas, M; Serve, H; Proschak, E; Lausen, J
Nature communications
5
3995
2014
Abstract anzeigen
The transcription factor Tal1 is a critical activator or repressor of gene expression in hematopoiesis and leukaemia. The mechanism by which Tal1 differentially influences transcription of distinct genes is not fully understood. Here we show that Tal1 interacts with the peptidylarginine deiminase IV (PADI4). We demonstrate that PADI4 can act as an epigenetic coactivator through influencing H3R2me2a. At the Tal1/PADI4 target gene IL6ST the repressive H3R2me2a mark triggered by PRMT6 is counteracted by PADI4, which augments the active H3K4me3 mark and thus increases IL6ST expression. In contrast, at the CTCF promoter PADI4 acts as a repressor. We propose that the influence of PADI4 on IL6ST transcription plays a role in the control of IL6ST expression during lineage differentiation of hematopoietic stem/progenitor cells. These results open the possibility to pharmacologically influence Tal1 in leukaemia. | Western Blotting | 24874575
 |
Single-Cell Profiling of Epigenetic Modifiers Identifies PRDM14 as an Inducer of Cell Fate in the Mammalian Embryo.
Burton, Adam, et al.
Cell Rep, (2013)
2013
Abstract anzeigen
Cell plasticity or potency is necessary for the formation of multiple cell types. The mechanisms underlying this plasticity are largely unknown. Preimplantation mouse embryos undergo drastic changes in cellular potency, starting with the totipotent zygote through to the formation of the pluripotent inner cell mass (ICM) and differentiated trophectoderm in the blastocyst. Here, we set out to identify and functionally characterize chromatin modifiers that define the transitions of potency and cell fate in the mouse embryo. Using a quantitative microfluidics approach in single cells, we show that developmental transitions are marked by distinctive combinatorial profiles of epigenetic modifiers. Pluripotent cells of the ICM are distinct from their differentiated trophectoderm counterparts. We show that PRDM14 is heterogeneously expressed in 4-cell-stage embryos. Forced expression of PRDM14 at the 2-cell stage leads to increased H3R26me2 and can induce a pluripotent ICM fate. Our results shed light on the epigenetic networks that govern cellular potency and identity in vivo. VIDEO ABSTRACT: | Immunocytochemistry | 24183668
 |
Protein arginine methyltransferase 7 regulates cellular response to DNA damage by methylating promoter histones H2A and H4 of the polymerase δ catalytic subunit gene, POLD1.
Karkhanis, V; Wang, L; Tae, S; Hu, YJ; Imbalzano, AN; Sif, S
The Journal of biological chemistry
287
29801-14
2011
Abstract anzeigen
Covalent modification of histones by protein arginine methyltransferases (PRMTs) impacts genome organization and gene expression. In this report, we show that PRMT7 interacts with the BRG1-based hSWI/SNF chromatin remodeling complex and specifically methylates histone H2A Arg-3 (H2AR3) and histone H4 Arg-3 (H4R3). To elucidate the biological function of PRMT7, we knocked down its expression in NIH 3T3 cells and analyzed global gene expression. Our findings show that PRMT7 negatively regulates expression of genes involved in DNA repair, including ALKBH5, APEX2, POLD1, and POLD2. Chromatin immunoprecipitation (ChIP) revealed that PRMT7 and dimethylated H2AR3 and H4R3 are enriched at target DNA repair genes in parental cells, whereas PRMT7 knockdown caused a significant decrease in PRMT7 recruitment and H2AR3/H4R3 methylation. Decreased PRMT7 expression also resulted in derepression of target DNA repair genes and enhanced cell resistance to DNA-damaging agents. Furthermore, we show that BRG1 co-localizes with PRMT7 on target promoters and that expression of a catalytically inactive form of BRG1 results in derepression of PRMT7 target DNA repair genes. Remarkably, reducing expression of individual PRMT7 target DNA repair genes showed that only the catalytic subunit of DNA polymerase, POLD1, was able to resensitize PRMT7 knock-down cells to DNA-damaging agents. These results provide evidence for the important role played by PRMT7 in epigenetic regulation of DNA repair genes and cellular response to DNA damage. | | 22761421
 |
The expression of myogenic microRNAs indirectly requires protein arginine methyltransferase (Prmt)5 but directly requires Prmt4.
Mallappa, C; Hu, YJ; Shamulailatpam, P; Tae, S; Sif, S; Imbalzano, AN
Nucleic acids research
39
1243-55
2010
Abstract anzeigen
Myogenic microRNAs are important regulators of muscle development and differentiation. To better understand the roles of chromatin-modifying and remodeling enzymes in the activation of myogenic microRNA expression, we have functionally analyzed two different protein arginine methyltransferases, Prmt5 and Prmt4, both of which have previously been implicated in the regulation of myogenic mRNA expression. Both Prmts are required for myogenic microRNA induction during differentiation. Prmt5 is indirectly required due to the necessity of Prmt5 for expression of the transcriptional regulator, myogenin, as ectopic expression of myogenin eliminates Prmt5 dependency. By contrast, Prmt4 binds to the upstream regulatory regions of myogenic microRNAs and is required for dimethylation of the Prmt4 substrate, H3R17, at microRNA regulatory sequences. Deletion of Prmt4 does not alter MyoD binding at myogenic microRNA regulatory sequences but prevents the binding of both myogenin and the Brg1 ATPase that catalyzes SWI/SNF-dependent chromatin remodeling, resulting in an inhibition of microRNA expression. | Western Blotting | 20947566
 |
Histone arginine methylation regulates pluripotency in the early mouse embryo.
Torres-Padilla, Maria-Elena, et al.
Nature, 445: 214-8 (2007)
2007
Abstract anzeigen
It has been generally accepted that the mammalian embryo starts its development with all cells identical, and only when inside and outside cells form do differences between cells first emerge. However, recent findings show that cells in the mouse embryo can differ in their developmental fate and potency as early as the four-cell stage. These differences depend on the orientation and order of the cleavage divisions that generated them. Because epigenetic marks are suggested to be involved in sustaining pluripotency, we considered that such developmental properties might be achieved through epigenetic mechanisms. Here we show that modification of histone H3, through the methylation of specific arginine residues, is correlated with cell fate and potency. Levels of H3 methylation at specific arginine residues are maximal in four-cell blastomeres that will contribute to the inner cell mass (ICM) and polar trophectoderm and undertake full development when combined together in chimaeras. Arginine methylation of H3 is minimal in cells whose progeny contributes more to the mural trophectoderm and that show compromised development when combined in chimaeras. This suggests that higher levels of H3 arginine methylation predispose blastomeres to contribute to the pluripotent cells of the ICM. We confirm this prediction by overexpressing the H3-specific arginine methyltransferase CARM1 in individual blastomeres and show that this directs their progeny to the ICM and results in a dramatic upregulation of Nanog and Sox2. Thus, our results identify specific histone modifications as the earliest known epigenetic marker contributing to development of ICM and show that manipulation of epigenetic information influences cell fate determination. | | 17215844
 |
Aberrant expression of CARM1, a transcriptional coactivator of androgen receptor, in the development of prostate carcinoma and androgen-independent status.
Hong, Heng, et al.
Cancer, 101: 83-9 (2004)
2004
| | 15221992
 |
Regulation of transcription by a protein methyltransferase.
Chen, D, et al.
Science, 284: 2174-7 (1999)
1998
Abstract anzeigen
The p160 family of coactivators, SRC-1, GRIP1/TIF2, and p/CIP, mediate transcriptional activation by nuclear hormone receptors. Coactivator-associated arginine methyltransferase 1 (CARM1), a previously unidentified protein that binds to the carboxyl-terminal region of p160 coactivators, enhanced transcriptional activation by nuclear receptors, but only when GRIP1 or SRC-1a was coexpressed. Thus, CARM1 functions as a secondary coactivator through its association with p160 coactivators. CARM1 can methylate histone H3 in vitro, and a mutation in the putative S-adenosylmethionine binding domain of CARM1 substantially reduced both methyltransferase and coactivator activities. Thus, coactivator-mediated methylation of proteins in the transcription machinery may contribute to transcriptional regulation. | | 10381882
 |