Beta-amyloid expression, release and extracellular deposition in aged rat brain slices.
Marksteiner, J; Humpel, C
Molecular psychiatry
13
939-52
2008
Abstract anzeigen
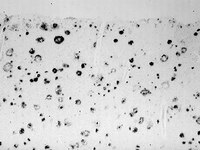
Alzheimer's disease (AD) is characterized by beta-amyloid plaques, tau pathology, cholinergic cell death and inflammation. The aim of this study was to investigate whether beta-amyloid is generated, released and extracellularly deposited in organotypic brain slices. In developing slices, no amyloid-precursor protein (APP) was detectable; however, there was a strong upregulation in aging slices. In such slices, rat beta-amyloid(1-42) and -(1-40) peptides were found using four sequence-specific antibodies. APP and beta-amyloid were expressed in neurons and to a lesser extent in astrocytes. Beta-amyloid was secreted into the medium. Beta-amyloid was located extracellularly when aging slices were incubated with medium at pH 6.0 including apolipoprotein E4 (ApoE4). It is concluded that aging organotypic brain slices express beta-amyloid and that acidosis induces cell death with efflux of beta-amyloid and extracellular depositions, which is triggered by ApoE4. This novel in vitro model may enable us to investigate further the pathological cascade for AD and may be useful to explore future therapeutics. | Immunohistochemistry | Rat | 17712316
 |
Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process.
Takashi Togo, Dennis W Dickson, Takashi Togo, Dennis W Dickson
Acta neuropathologica
104
398-402
2002
Abstract anzeigen
Tau-immunoreactive astrocytes in progressive supranuclear palsy (PSP) have a distinctive morphology and are referred to as tufted astrocytes (TA). We hypothesized that TA may be a degenerative change in reactive astrocytes. To test this hypothesis we examined the relationship of TA to gliosis in PSP. We first examined the distribution of gliosis [glial fibrillary acid protein (GFAP)-positive astrocytes], TA, neurofibrillary tangles (NFT) and pretangles in brain sections of neuropathologically pure PSP cases. Second, we examined PSP cases complicated by infarcts or Alzheimer's disease, since these cases would have reactive astrocytes associated with lesions. We used double immunostaining for GFAP and tau for cases with vascular lesions, and triple immunostaining for GFAP, tau and beta-amyloid protein for sections with senile plaques. There was no correlation between the distribution of gliosis and TA, with gliosis prominent in globus pallidus and subthalamic nucleus, and TA prominent in motor cortex and striatum. On the other hand, gliosis paralleled the distribution of NFT, but not the distribution of pretangles, suggesting that NFT contributes to gliosis in PSP. Although reactive astrocytes were present around infarcts and senile plaques, TA were not associated with these lesions. Tau accumulation in astrocytes in PSP was not preferential to (and was actually independent of) reactive astrocytes. This is consistent with the notion that tau accumulation in astrocytes is a degenerative rather than reactive process. Unlike NFT, astrocytic degeneration does not seem to contribute to gliosis or neuronal loss in PSP, and its clinical significance remains unclear. | | | 12200627
 |