Steritest™ NEO Accessories
Recommended Products
개요
사양
주문 정보
Documentation
References
| Reference overview | Pub Med ID |
|---|---|
| The Effect of Vaporous Phase Hydrogen Peroxide on Sterility Test Devices Roche Lentine, Kerry and Robert J. Keller; Pharmaceutical Technology Europe, February 2003: 31- 42. Pharmaceutical Technology Europe, February 2003: 31- 42. 2003 | The Effect of Vaporous Phase Hydrogen Peroxide on Sterility Test Devices
 |
관련 제품 및 어플리케이션
제품 패밀리

Steritest™ NEO Devices for Products Without Antimicrobial AgentsBlue canister base. Mixed esters of cellulose membrane. This membrane provides an optimal filtration flow rate for standard products. Canisters material: SAN (Styrene-Acrylo Nitrile)더 보기 >> |

Steritest™ NEO Devices for Antibiotics and Products with Antimicrobial ActivityRed canister base with specific drain design. Low binding PVDF Durapore® membrane. This optimizes the rinsing of products that inhibit microbial growth. Canisters material: SAN더 보기 >> |

Steritest™ NEO Device for products requiring increased Chemical CompatibilityGreen canister base with specific drain design. Low binding PVDF Durapore® membrane optimize the rinsing of products that inhibit microbial growth. Canisters material: polyamide polymer.더 보기 >> |

Steritest™ EZ Devices Double-PackedIncrease your test method reliability with our gamma sterilized sterility testing device더 보기 >> |

Sterility TestingCountless configurations더 보기 >> |
관련 제품: Application Facete
| Sterility Testing |
관련 제품: Brand Facete
| Steridilutor® |
| Steritest™ |
카테고리
| Industrial Microbiology > Sterility Testing > Membrane Filtration Sterility Test |
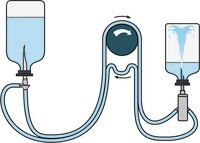
Sample Preparation and Dilution
- Tubing and needle assembly to dissolve powders, for dilution and pool products in vials
- To be used for difficult to dissolve powders, dilution and pooling of viscous products in vials as well as antibiotics (to reduce the contact time with the filtration membrane)
- The expansion chamber vents residual vacuum or pressure from the vials without after-drip or contamination risk
- Small diameter double needle connects test product to diluent
- Diluted product subsequently filtered with suitable Steritest™ NEO canisters
Steridilutor® NEO devices for liquids and soluble powders in vials - without expansion chamber TZV000010
Steridilutor® NEO devices for liquids and soluble powders in vials - with expansion chamber TZVC00010
Liquid Transfer
- Tubing and needle assembly for transfer of liquids from ampoules or vials to a diluent vial with septum
- Diluted products subsequently tested with suitable Steritest™ NEO canister
Steridilutor® NEO devices for liquid transfer TZA000010
Sterile Vent Needles
- Single needle vented with PTFE 0.22 μm membrane
- For venting glass vials with septa and rigid plastic vials
- For venting of media bottles during the direct inoculation method
- For sterility and growth promotion qualification of media batches












