532722 Sigma-AldrichNurr1 Activator, IP7e - CAS 500164-74-9 - Calbiochem
A cell-permeable, blood-brain barrier permeable, potent activator of Nurr1/NR4A2-dependent transcription activity in (EC₅₀ = 3.9 nM in MN90 cell line).
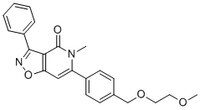
More>> A cell-permeable, blood-brain barrier permeable, potent activator of Nurr1/NR4A2-dependent transcription activity in (EC₅₀ = 3.9 nM in MN90 cell line). Less<<Synonyms: (6-(4-((2-Methoxyethoxy)methyl)phenyl)-5-methyl-3-phenylisoxazolo(4,5-c)pyridin-4(5H)-one), 6-(4-((2-Methoxyethoxy)methyl)phenyl)-5-methyl-3-phenyl[1,2]oxazolo[4,5-c]pyridin-4(5H)-one, Isoxazolo-Pyridinone 7e, NR4A2 Activator
Recommended Products
개요
| Replacement Information |
|---|
주요 사양표
| CAS # | Empirical Formula |
|---|---|
| 500164-74-9 | C₂₃H₂₂N₂O₄ |
가격 및 재고여부
| 카탈로그 번호 | 재고 정보 | 패킹 | 포장 단위 | 가격(VAT 별도) | 수량 | |
|---|---|---|---|---|---|---|
| 5.32722.0001 |
|
Glass bottle | 10 mg |
|
— |
| References | |
|---|---|
| References | Montarolo, F., 2014. PLoS One. 9, e108791. Hintermann, S., et al. 2007. Bioorg. Med. Chem. Lett. 17, 193. |
| Product Information | |
|---|---|
| CAS number | 500164-74-9 |
| Form | Off-white solid |
| Hill Formula | C₂₃H₂₂N₂O₄ |
| Chemical formula | C₂₃H₂₂N₂O₄ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Nurr1/NR4A2 |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 카탈로그 번호 | GTIN |
| 5.32722.0001 | 04055977281866 |
Documentation
Nurr1 Activator, IP7e - CAS 500164-74-9 - Calbiochem MSDS
| 타이틀 |
|---|
References
| 참고문헌 보기 |
|---|
| Montarolo, F., 2014. PLoS One. 9, e108791. Hintermann, S., et al. 2007. Bioorg. Med. Chem. Lett. 17, 193. |
Brochure
| Title |
|---|
| Neuroscience Solutions for productive research |