Neonatal exposure to sucralose does not alter biochemical markers of neuronal development or adult behavior.
Henrik Viberg,Anders Fredriksson
Nutrition (Burbank, Los Angeles County, Calif.)
27
2011
요약 표시
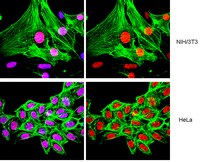
Sucralose, a high-intensity sweetener, has been approved as a general-purpose sweetener in all food since the late 1990s. Due to its good taste and physiochemical profile, its use has increased and sucralose is considered a way of managing health and an option to improve the quality of life in the diabetic population. Recently high concentrations of sucralose have been found in the environment. Other environmental pollutants have been shown to induce neurotoxic effects when administered during a period of rapid brain growth and development. This period of rapid brain growth and development is postnatal in mice and rats, spanning the first 3-4 wk of life, reaching its peak around postnatal day 10, whereas in humans, brain growth and development is perinatal. The proteins calcium/calmodulin-dependent protein kinase II, growth-associated protein-43, synaptophysin, and tau play important roles during brain growth and development. | 20116214
 |
Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator.
Tanner, K G, et al.
J. Biol. Chem., 274: 18157-60 (1999)
1999
요약 표시
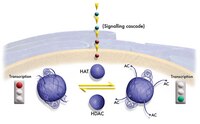
Within chromatin, reversible acetylation of core histones is critical for transcriptional activation of eukaryotic target genes. The recent identification of intrinsic histone acetyltransferase (HAT) catalytic activity from a number of transcriptional co-activators (including yeast GCN5, p300/CBP, P/CAF, and TAFII250), has underscored the importance of protein acetylation in transcriptional control. The GCN5 family is the prototype for a diverse group of at least four distinct human HATs families. Although there is now a clear link between in vivo HAT catalytic activity and gene activation, little is known about the molecular mechanisms of histone acetylation. Herein, we report the first detailed biochemical study that probes the catalytic mechanism and the function of invariant glutamic acid 173 within the GCN5 family of HATs. Our results suggest that the HAT reaction involves the formation of a ternary complex (histones, acetyl-CoA, and enzyme) where the epsilon-amino group of histone lysine residues directly attacks the bound acetyl-CoA. The acetylation reaction requires deprotonation of the epsilon-amino group prior to nucleophilic attack. Employing site-directed mutagenesis, chemical modification, steady-state, and pH-dependent rate analysis, it is demonstrated that glutamic acid 173 is an essential catalytic residue, acting as a general base catalyst by deprotonating the histone substrate. | 10373413
 |