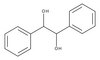
Edaravone, a ROS scavenger, ameliorates photoreceptor cell death after experimental retinal detachment.
Roh M, Murakami Y, Thanos A, Vavvas D, Miller JW
Invest Ophthalmol Vis Sci
2011
요약 표시
Purpose. To investigate whether edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a free radical scavenger is neuroprotective against photoreceptor cell death in a rat model of retinal detachment (RD). Methods. RD was induced in adult Brown Norway rats by subretinal injection of sodium hyaluronate. Edaravone (3 mg/kg, 5 mg/kg, 10 mg/kg) or physiologic saline were administered intraperitoneally once a day until sacrifice on day 3 or 5. Oxidative stress in the retina was assessed by 4-hydroxynonenal staining or ELISA for protein carbonyl content. Photoreceptor death was assessed by TUNEL and measurement of the outer nuclear layer thickness . Western blot analysis and caspase activity assays were performed. Inflammatory cytokine secretion and inflammatory cell infiltration were evaluated by ELISA and immunostaining, respectively. Results. RD resulted in increased generation of ROS. Treatment with 5 mg/kg edaravone significantly reduced ROS level along with decrease in TUNEL positive cells in the photoreceptor layer. Caspase assay also confirmed decreased activation of caspases -3,-,8, and -9 in RD treated with edaravone. The level of the anti-apoptotic Bcl-2 was increased in detached retinas after edaravone treatment, whereas the levels of the stress-activated p-ERK1/2 were decreased. Additionally, edaravone treatment resulted in a significant decrease in the levels of TNF-α, MCP-1 and macrophage infiltration. Conclusions. Oxidative stress plays an important role in the photoreceptor cell death after RD. Edaravone treatment may aid in preventing photoreceptor cell death following RD by suppressing ROS-induced photoreceptor damage. | 21310909
 |
Neuroprotective effect of Ro5-4864 following brain injury.
Jean F Soustiel,Menashe Zaaroor,Eugene Vlodavsky,Leo Veenman,Abraham Weizman,Moshe Gavish
Experimental neurology
214
2008
요약 표시
The 18 kDa translocator protein (TSPO) is a protein complex located at the outer mitochondrial membrane and interacting with the mitochondrial permeability transition pore (mPTP), indicating its involvement in the control of mPTP opening. We intended to explore the effect of TSPO ligands, PK 11195 and Ro5-4864 on apoptosis in a rat model of cortical injury. Sprague-Dawley rats received a daily intraperitoneal injection of dimethylsulfoxide (vehicle), PK 11195, or Ro5-4864, starting 2 days prior the injury and a third injection after the injury. At 6 weeks, immunohistochemistry analysis showed that Ro5-4864 resulted in a significant increase in the number of surviving neurons and in the density of the neurofilament network in the perilesional cortex in comparison with animals of the vehicle and PK 11195 groups. In tissue samples dissected from the injured area, Ro5-4864 caused a significant reduction in activation of caspases 3 and 9 but not of caspase 8 in comparison with the vehicle and PK 11195 groups. In addition, measurements of transmembrane mitochondrial potential of mitochondria (Deltapsi(M)) isolated from normal rat brain showed that loss of Deltapsi(M) induced by recombinant Bax could be significantly reduced by Ro5-4864 in a concentration-dependent manner. Our findings indicate that the neuroprotective effect shown by Ro5-4864 in the present model of brain injury involves the mitochondrial pathway of apoptosis modulation of mPTP. | 18789929
 |
The effect of oxygenation level on cerebral post-traumatic apoptotsis is modulated by the 18-kDa translocator protein (also known as peripheral-type benzodiazepine receptor) in a rat model of cortical contusion.
J F Soustiel,E Palzur,E Vlodavsky,L Veenman,M Gavish
Neuropathology and applied neurobiology
34
2008
요약 표시
Hyperbaric hyperoxia has been shown to reduce apoptosis in brain injury. As the 18-kDa translocator protein (TSPO), also known as peripheral-type benzodiazepine receptor, is closely associated with the mitochondrial transition pore and because of its role in mitochondrial respiration and apoptosis, we hypothesized that reduction of apoptosis by hyperoxia may involve the TSPO. | 17973904
 |