208743 Sigma-AldrichCalpain Inhibitor XI - CAS 145731-49-3 - Calbiochem
The Calpain Inhibitor XI, also referenced under CAS 145731-49-3, controls the biological activity of Calpain. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
More>> The Calpain Inhibitor XI, also referenced under CAS 145731-49-3, controls the biological activity of Calpain. This small molecule/inhibitor is primarily used for Protease Inhibitors applications. Less<<Synonyms: Z-L-Abu-CONH(CH₂)₃-morpholine
Recommended Products
개요
| Replacement Information |
|---|
주요 사양표
| CAS # | Empirical Formula |
|---|---|
| 145731-49-3 | C₂₆H₄₀N₄O₆ |
가격 및 재고여부
| 카탈로그 번호 | 재고 정보 | 패킹 | 포장 단위 | 가격(VAT 별도) | 수량 | |
|---|---|---|---|---|---|---|
| 208743-5MGCN |
|
Plastic ampoule | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 145731-49-3 |
| ATP Competitive | N |
| Form | White to off-white solid |
| Formulation | Supplied as a trifluoroacetate salt. |
| Hill Formula | C₂₆H₄₀N₄O₆ |
| Chemical formula | C₂₆H₄₀N₄O₆ |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
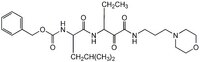
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | calpain 1, calpain 2 |
| Primary Target K<sub>i</sub> | 140 nM and 41 nM, against calpain-1 and -2, respectively |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Peptide Sequence | Z-Leu-Abu-CONH(CH₂)₃-morpholine (Abu = α-aminobutyric acid) |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 카탈로그 번호 | GTIN |
| 208743-5MGCN | 04055977202809 |
Documentation
Calpain Inhibitor XI - CAS 145731-49-3 - Calbiochem MSDS
| 타이틀 |
|---|
Calpain Inhibitor XI - CAS 145731-49-3 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 208743 |
References
| 참고문헌 보기 |
|---|
| Blomgren, K., et al. 2001. J. Biol. Chem. 276, 10191. DeBiasi, R.L., et al. 2001. J. Virol. 75, 351. Saatman, K.E., et al. 2000. J. Cereb. Blood Flow Metab. 20, 66. Stelmasiak, Z., et al. 2000. Med. Sci. Monit. 6, 426. Blomgren, K., et al. 1999. J. Biol. Chem. 274, 14046. James, T., et al. 1998. J. Neurosci. Res. 51, 218. Li, Z., et al. 1996. J. Med. Chem. 39, 4089. Saatman, K.E, et al. 1996. Proc. Natl. Acad. Sci. USA 93, 3428. Bartus, R.T., et al. 1995. Neurol. Res. 17, 249. Bartus, R.T., et al. 1994. Stroke 25, 2265. |