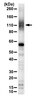
Functional interactions between the LRP6 WNT co-receptor and folate supplementation.
Gray, JD; Nakouzi, G; Slowinska-Castaldo, B; Dazard, JE; Rao, JS; Nadeau, JH; Ross, ME
Human molecular genetics
19
4560-72
2010
요약 표시
Crooked tail (Cd) mice bear a gain-of-function mutation in Lrp6, a co-receptor for canonical WNT signaling, and are a model of neural tube defects (NTDs), preventable with dietary folic acid (FA) supplementation. Whether the FA response reflects a direct influence of FA on LRP6 function was tested with prenatal supplementation in LRP6-deficient embryos. The enriched FA (10 ppm) diet reduced the occurrence of birth defects among all litters compared with the control (2 ppm FA) diet, but did so by increasing early lethality of Lrp6(-/-) embryos while actually increasing NTDs among nulls alive at embryonic days 10-13 (E10-13). Proliferation in cranial neural folds was reduced in homozygous Lrp6(-/-) mutants versus wild-type embryos at E10, and FA supplementation increased proliferation in wild-type but not mutant neuroepithelia. Canonical WNT activity was reduced in LRP6-deficient midbrain-hindbrain at E9.5, demonstrated in vivo by a TCF/LEF-reporter transgene. FA levels in media modulated the canonical WNT response in NIH3T3 cells, suggesting that although FA was required for optimal WNT signaling, even modest FA elevations attenuated LRP5/6-dependent canonical WNT responses. Gene expression analysis in embryos and adults showed striking interactions between targeted Lrp6 deficiency and FA supplementation, especially for mitochondrial function, folate and methionine metabolism, WNT signaling and cytoskeletal regulation that together implicate relevant signaling and metabolic pathways supporting cell proliferation, morphology and differentiation. We propose that FA supplementation rescues Lrp6(Cd/Cd) fetuses by normalizing hyperactive WNT activity, whereas in LRP6-deficient embryos, added FA further attenuates reduced WNT activity, thereby compromising development. | 20843827
 |
Selective cortical interneuron and GABA deficits in cyclin D2-null mice.
Glickstein, SB; Moore, H; Slowinska, B; Racchumi, J; Suh, M; Chuhma, N; Ross, ME
Development (Cambridge, England)
134
4083-93
2007
요약 표시
In contrast to cyclin D1 nulls (cD1-/-), mice without cyclin D2 (cD2-/-) lack cerebellar stellate interneurons; the reason for this is unknown. In the present study in cortex, we found a disproportionate loss of parvalbumin (PV) interneurons in cD2-/- mice. This selective reduction in PV subtypes was associated with reduced frequency of GABA-mediated inhibitory postsynaptic currents in pyramidal neurons, as measured by voltage-clamp recordings, and increased cortical sharp activity in the EEGs of awake-behaving cD2-/- mice. Cell cycle regulation was examined in the medial ganglionic eminence (MGE), the major source of PV interneurons in mouse brain, and differences between cD2-/- and cD1-/- suggested that cD2 promotes subventricular zone (SVZ) divisions, exerting a stronger inhibitory influence on the p27 Cdk-inhibitor (Cdkn1b) to delay cell cycle exit of progenitors. We propose that cD2 promotes transit-amplifying divisions in the SVZ and that these ensure proper output of at least a subset of PV interneurons. | 17965053
 |