Histone H4 lysine 20 of Saccharomyces cerevisiae is monomethylated and functions in subtelomeric silencing.
Edwards, CR; Dang, W; Berger, SL
Biochemistry
50
10473-83
2011
요약 표시
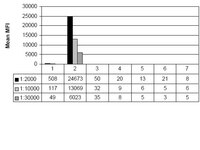
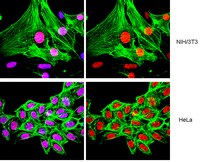
Histones undergo post-translational modifications that are linked to important biological processes. Previous studies have indicated that lysine methylation correlating with closed or repressive chromatin is absent in the budding yeast Saccharomyces cerevisiae, including at H4 lysine 20 (K20). Here we provide functional evidence for H4 K20 monomethylation (K20me1) in budding yeast. H4 K20me1 is detectable on endogenous H4 by western analysis using methyl-specific antibodies, and the signal is abrogated by H4 K20 substitutions and by competition with H4 K20me1 peptides. Using chromatin immunoprecipitation, we show that H4 K20me1 levels are highest at heterochromatic locations, including subtelomeres, the silent mating type locus, and rDNA repeats, and lowest at centromeres within euchromatin. Further, an H4 K20A substitution strongly reduced heterochromatic reporter silencing at telomeres and the silent mating type locus and led to an increase in subtelomeric endogenous gene expression. The correlation between the location of H4 K20me1 and the effect of the H4 K20A substitution suggests that this modification plays a repressive function. Our findings reveal the first negative regulatory histone methylation in budding yeast and indicate that H4 K20me1 is evolutionarily conserved from simple to complex eukaryotes. | 21985125
 |
L3MBTL1, a histone-methylation-dependent chromatin lock.
Trojer, Patrick, et al.
Cell, 129: 915-28 (2007)
2007
요약 표시
Distinct histone lysine methylation marks are involved in transcriptional repression linked to the formation and maintenance of facultative heterochromatin, although the underlying mechanisms remain unclear. We demonstrate that the malignant-brain-tumor (MBT) protein L3MBTL1 is in a complex with core histones, histone H1b, HP1gamma, and Rb. The MBT domain is structurally related to protein domains that directly bind methylated histone residues. Consistent with this, we found that the L3MBTL1 MBT domains compact nucleosomal arrays dependent on mono- and dimethylation of histone H4 lysine 20 and of histone H1b lysine 26. The MBT domains bind at least two nucleosomes simultaneously, linking repression of transcription to recognition of different histone marks by L3MBTL1. Consistently, L3MBTL1 was found to negatively regulate the expression of a subset of genes regulated by E2F, a factor that interacts with Rb. | 17540172
 |