WNT3A gene expression is associated with isolated Hirschsprung disease polymorphism and disease status.
Chen, D; Mi, J; Liu, X; Zhang, J; Wang, W; Gao, H
International journal of clinical and experimental pathology
7
1359-68
2014
요약 표시
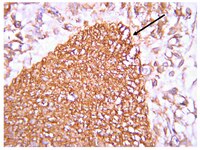
WNT3A has been regarded as an activator of the canonical Wnt signaling pathway. It has been found Wnt signaling pathway is closely related with embrionic development and Hirschsprung disease (HSCR). A common haplotype consisting of minor SNPs alleles located in the WNT3A gene has been described as a risk factor for various genetic disorders. However, whether WNT3A contributes to the onset of HSCR has not been identified. The present study aims to detect the interactions of genetic variations in the WNT3A gene and examine the biological expression levels with Hirschsprung disease (HSCR) in the Chinese people. We analyzed WNT3A gene (rs61743220, rs192966556 and rs145882986) variants in the whole blood samples from HSCR patients and normal children (control groups). WNT3A expression was also examined by quantitative real-time PCR (qRT-PCR), western blotting and immunostaining. Consequently, when rs192966556 and rs145882986 alleles of the WNT3A gene lack the SNPs, they are especially associated with a greater risk of HSCR (OR [95% confidence interval]=1.791, p=0.001; OR [95% confidence interval]=1.556, p=0.003, respectively). The mRNA and protein expressions of WNT3A were higher in the aganglionic colon segment tissues than in the normal ganglionic segments tissues. Immunostaining indicates that the staining of WNT3A was much stronger (brown) in the aganglionic colon segment tissues than that in the normal ganglionic colon segment tissues (colorless or light yellow) in the mucous layer and muscular layer. Although preliminary, these results suggest that WNT3A may play an important role in the pathogenesis of HSCR. | Western Blotting | 24817932
 |
Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease.
Boulter, L; Govaere, O; Bird, TG; Radulescu, S; Ramachandran, P; Pellicoro, A; Ridgway, RA; Seo, SS; Spee, B; Van Rooijen, N; Sansom, OJ; Iredale, JP; Lowell, S; Roskams, T; Forbes, SJ
Nature medicine
18
572-9
2012
요약 표시
During chronic injury a population of bipotent hepatic progenitor cells (HPCs) become activated to regenerate both cholangiocytes and hepatocytes. Here we show in human diseased liver and mouse models of the ductular reaction that Notch and Wnt signaling direct specification of HPCs via their interactions with activated myofibroblasts or macrophages. In particular, we found that during biliary regeneration, expression of Jagged 1 (a Notch ligand) by myofibroblasts promoted Notch signaling in HPCs and thus their biliary specification to cholangiocytes. Alternatively, during hepatocyte regeneration, macrophage engulfment of hepatocyte debris induced Wnt3a expression. This resulted in canonical Wnt signaling in nearby HPCs, thus maintaining expression of Numb (a cell fate determinant) within these cells and the promotion of their specification to hepatocytes. By these two pathways adult parenchymal regeneration during chronic liver injury is promoted. | Immunohistochemistry | 22388089
 |
Heparan sulfate 6-O-endosulfatases (Sulfs) coordinate the Wnt signaling pathways to regulate myoblast fusion during skeletal muscle regeneration.
Tran, TH; Shi, X; Zaia, J; Ai, X
The Journal of biological chemistry
287
32651-64
2012
요약 표시
Skeletal muscle regeneration is mediated by satellite cells (SCs). Upon injury, SCs undergo self-renewal, proliferation, and differentiation into myoblasts followed by myoblast fusion to form new myofibers. We previously showed that the heparan sulfate (HS) 6-O-endosulfatases (Sulf1 and -2) repress FGF signaling to induce SC differentiation during muscle regeneration. Here, we identify a novel role of Sulfs in myoblast fusion using a skeletal muscle-specific Sulf double null (Sulf(SK)-DN) mouse. Regenerating Sulf(SK)-DN muscles exhibit reduced canonical Wnt signaling and elevated non-canonical Wnt signaling. In addition, we show that Sulfs are required to repress non-canonical Wnt signaling to promote myoblast fusion. Notably, skeletal muscle-relevant non-canonical Wnt ligands lack HS binding capacity, suggesting that Sulfs indirectly repress this pathway. Mechanistically, we show that Sulfs reduce the canonical Wnt-HS binding and regulate colocalization of the co-receptor LRP5 with caveolin3. Therefore, Sulfs may increase the bioavailability of canonical Wnts for Frizzled receptor and LRP5/6 interaction in lipid raft, which may in turn antagonize non-canonical Wnt signaling. Furthermore, changes in subcellular distribution of active focal adhesion kinase (FAK) are associated with the fusion defect of Sulf-deficient myoblasts and upon non-canonical Wnt treatment. Together, our findings uncover a critical role of Sulfs in myoblast fusion by promoting antagonizing canonical Wnt signaling activities against the noncanonical Wnt pathway during skeletal muscle regeneration. | | 22865881
 |
Regulation of Wnt signaling by nociceptive input in animal models.
Shi, Y; Yuan, S; Li, B; Wang, J; Carlton, SM; Chung, K; Chung, JM; Tang, SJ
Molecular pain
8
47
2012
요약 표시
Central sensitization-associated synaptic plasticity in the spinal cord dorsal horn (SCDH) critically contributes to the development of chronic pain, but understanding of the underlying molecular pathways is still incomplete. Emerging evidence suggests that Wnt signaling plays a crucial role in regulation of synaptic plasticity. Little is known about the potential function of the Wnt signaling cascades in chronic pain development.Fluorescent immunostaining results indicate that β-catenin, an essential protein in the canonical Wnt signaling pathway, is expressed in the superficial layers of the mouse SCDH with enrichment at synapses in lamina II. In addition, Wnt3a, a prototypic Wnt ligand that activates the canonical pathway, is also enriched in the superficial layers. Immunoblotting analysis indicates that both Wnt3a a β-catenin are up-regulated in the SCDH of various mouse pain models created by hind-paw injection of capsaicin, intrathecal (i.t.) injection of HIV-gp120 protein or spinal nerve ligation (SNL). Furthermore, Wnt5a, a prototypic Wnt ligand for non-canonical pathways, and its receptor Ror2 are also up-regulated in the SCDH of these models.Our results suggest that Wnt signaling pathways are regulated by nociceptive input. The activation of Wnt signaling may regulate the expression of spinal central sensitization during the development of acute and chronic pain. | Immunofluorescence | 22713358
 |
A low-protein diet supplemented with ketoacids plays a more protective role against oxidative stress of rat kidney tissue with 5/6 nephrectomy than a low-protein diet alone.
Xiang Gao,Jianxiang Wu,Zheyi Dong,Can Hua,Huimin Hu,Changlin Mei
The British journal of nutrition
103
2010
요약 표시
Dietary protein restriction is one major therapy in chronic kidney disease (CKD), and ketoacids have been evaluated in CKD patients during restricted-protein diets. The objective of the present study was to compare the efficacy of a low-protein diet supplemented with ketoacids (LPD+KA) and a low-protein diet alone (LPD) in halting the development of renal lesions in CKD. 5/6 Nephrectomy Sprague-Dawley rats were randomly divided into three groups, and fed with either 22 % protein (normal-protein diet; NPD), 6 % protein (LPD) or 5 % protein plus 1 % ketoacids (LPD+KA) for 24 weeks. Sham-operated rats were used as controls. Each 5/6 nephrectomy group included fifteen rats and the control group included twelve rats. Proteinuria, decreased renal function, glomerular sclerosis and tubulointerstitial fibrosis were found in the remnant kidneys of the NPD group. Protein restriction ameliorated these changes, and the effect was more obvious in the LPD+KA group after 5/6 nephrectomy. Lower body weight and serum albumin levels were found in the LPD group, indicating protein malnutrition. Lipid and protein oxidative products were significantly increased in the LPD group compared with the LPD+KA group. These findings indicate that a LPD supplemented with ketoacids is more effective than a LPD alone in protecting the function of remnant kidneys from progressive injury, which may be mediated by ketoacids ameliorating protein malnutrition and oxidative stress injury in remnant kidney tissue. | | 19878616
 |