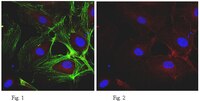
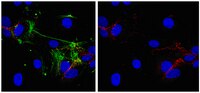
Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability.
Corada, M; Liao, F; Lindgren, M; Lampugnani, MG; Breviario, F; Frank, R; Muller, WA; Hicklin, DJ; Bohlen, P; Dejana, E
Blood
97
1679-84
2001
요약 표시
Vascular endothelial cadherin (VE-cadherin) is an endothelial cell-specific cadherin that plays an important role in the control of vascular organization. Blocking VE-cadherin antibodies strongly inhibit angiogenesis, and inactivation of VE-cadherin gene causes embryonic lethality due to a lack of correct organization and remodeling of the vasculature. Hence, inhibitors of VE-cadherin adhesive properties may constitute a tool to prevent tumor neovascularization. In this paper, we tested different monoclonal antibodies (mAbs) directed to human VE-cadherin ectodomain for their functional activity. Three mAbs (Cad 5, BV6, BV9) were able to increase paracellular permeability, inhibit VE-cadherin reorganization, and block angiogenesis in vitro. These mAbs could also induce endothelial cell apoptosis in vitro. Two additional mAbs, TEA 1.31 and Hec 1.2, had an intermediate or undetectable activity, respectively, in these assays. Epitope mapping studies show that BV6, BV9, TEA 1.31, and Hec 1.2 bound to a recombinant fragment spanning the extracellular juxtamembrane domains EC3 through EC4. In contrast, Cad 5 bound to the aminoterminal domain EC1. By peptide scanning analysis and competition experiments, we defined the sequences TIDLRY located on EC3 and KVFRVDAETGDVFAI on EC1 as the binding domain of BV6 and Cad 5, respectively. Overall, these results support the concept that VE-cadherin plays a relevant role on human endothelial cell properties. Antibodies directed to the extracellular domains EC1 but also EC3-EC4 affect VE-cadherin adhesion and clustering and alter endothelial cell permeability, apoptosis, and vascular structure formation. | 11238107
 |
Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin).
Caveda, L; Martin-Padura, I; Navarro, P; Breviario, F; Corada, M; Gulino, D; Lampugnani, MG; Dejana, E
The Journal of clinical investigation
98
886-93
1996
요약 표시
Endothelial cell proliferation is inhibited by the establishment of cell to cell contacts. Adhesive molecules at junctions could therefore play a role in transferring negative growth signals. The transmembrane protein VE-cadherin (vascular endothelial cadherin/cadherin-S) is selectively expressed at intercellular clefts in the endothelium. The intracellular domain interacts with cytoplasmic proteins called catenins that transmit the adhesion signal and contribute to the anchorage of the protein to the actin cytoskeleton. Transfection of VE-cadherin in both Chinese hamster ovary (CHO) and L929 cells confers inhibition of cell growth. Truncation of VE-cadherin cytoplasmic region, responsible for linking catenins, does not affect VE-cadherin adhesive properties but abolishes its effect on cell growth. Seeding human umbilical vein endothelial cells or VE-cadherin transfectants on a recombinant VE-cadherin amino-terminal fragment inhibited their proliferation. These data show that VE-cadherin homotypic engagement at junctions participates in density dependent inhibition of cell growth. This effect requires both the extracellular adhesive domain and the intracellular catenin binding region of the molecule. | 8770858
 |
Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin.
Breviario, F; Caveda, L; Corada, M; Martin-Padura, I; Navarro, P; Golay, J; Introna, M; Gulino, D; Lampugnani, MG; Dejana, E
Arteriosclerosis, thrombosis, and vascular biology
15
1229-39
1995
요약 표시
Human vascular endothelial cadherin (VE-cadherin, 7B4/cadherin-5) is an endothelial-specific cadherin localized at the intercellular junctions. To directly investigate the functional role of this molecule we cloned the full-length cDNA from human endothelial cells and transfected its coding region into Chinese hamster ovary cells. The product of the transfected cDNA had the same molecular weight as the natural VE-cadherin in human endothelial cells, and reacted with several VE-cadherin mouse monoclonal antibodies. Furthermore, it selectively concentrated at intercellular junctions, where it codistributed with alpha-catenin. VE-cadherin conferred adhesive properties to transfected cells. It mediated homophilic, calcium-dependent aggregation and cell-to-cell adhesion. In addition, it decreased intercellular permeability to high-molecular weight molecules and reduced cell migration rate across a wounded area. Thus, VE-cadherin may exert a relevant role in endothelial cell biology through control of the cohesion and organization of the intercellular junctions. | 7627717
 |
A novel endothelial-specific membrane protein is a marker of cell-cell contacts.
Lampugnani, MG; Resnati, M; Raiteri, M; Pigott, R; Pisacane, A; Houen, G; Ruco, LP; Dejana, E
The Journal of cell biology
118
1511-22
1992
요약 표시
mAbs were raised in mice against cultured human endothelial cells (EC) and screened by indirect immunofluorescence for their ability to stain intercellular contacts. One mAb denoted 7B4 was identified which, out of many cultured cell types, specifically decorated cultured human EC. The antigen recognized by mAb 7B4 is bound at the appositional surfaces of cultured EC only as they become confluent and is stably expressed at intercellular boundaries of confluent monolayers. EC recognition specificity was maintained when the antibody was assayed by immuno-histochemistry in tissue sections of many normal and malignant tissues and in blood vessels of different size and type. The antigen recognized by 7B4 was enriched at EC intercellular boundaries similarly in vitro and in situ. In vitro, addition of mAb 7B4 to confluent EC increased permeation of macromolecules across monolayers even without any obvious changes of cell morphology. In addition, when EC permeability was increased by agents such as thrombin, elastase, and TNF/gamma IFN, its distribution pattern at intercellular contact rims was severely altered. mAb 7B4 immunoprecipitated a major protein of 140 kD from metabolically and surface-labeled cultured EC extracts which appeared to be an integral membrane glycoprotein. On the basis of its distribution in cultured cells and in tissues in situ, 7B4 antigen is distinct from other described EC proteins enriched at intercellular contacts. NH2-terminal sequencing of the antigen, immunopurified from human placenta, and sequencing of peptides from tryptic peptide maps revealed identity to the cDNA deduced sequence of a recently identified new member of the cadherin family (Suzuki, S., K. Sano, and H. Tanihara. 1991. Cell Regul. 2:261-270.) These data indicate that 7B4 antigen is an endothelial-specific cadherin that plays a role in the organization of lateral endothelial junctions and in the control of permeability properties of vascular endothelium. | 1522121
 |