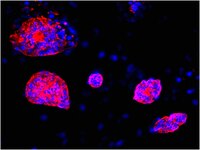
Efficient derivation of embryonic stem cells from nuclear transfer and parthenogenetic embryos derived from cryopreserved oocytes.
Sung LY, Chang CC, Amano T, Lin CJ, Amano M, Treaster SB, Xu J, Chang WF, Nagy ZP, Yang X, Tian XC
Cell Reprogram
12
203-11.
2010
요약 표시
Deriving histocompatible embryonic stem (ES) cells by somatic cell nuclear transfer (SCNT) and parthenogenetic activation (PA) requires fresh oocytes, which prevents their applications in humans. Here, we evaluated the efficiency of deriving ES cells from mature metaphase II (MII) and immature metaphase I (MI) vitrified oocytes, by PA or SCNT, in a mouse model. We successfully generated ES cell lines from PA (MII and MI) and SCNT (MII and MI) blastocysts. These cell lines expressed genes and antigens characteristic of pluripotent ES cells and produced full-term pups upon tetraploid embryo complementation. This study established an animal model for efficient generation of patient-specific ES cell lines using cryopreserved oocytes. This is a major step forward in the application of therapeutic cloning and parthenogenetic technology in human regenerative medicine and will serve as an important alternative to the iPS cell technology in countries/regions where these technologies are permitted. | 20677934
 |
Establishment and characterization of embryonic stem-like cells from porcine somatic cell nuclear transfer blastocysts.
Kim S, Kim JH, Lee E, Jeong YW, Hossein MS, Park SM, Park SW, Lee JY, Jeong YI, Kim HS, Kim YW, Hyun SH, Hwang WS
Zygote
18
93-101. Epub 2010 Mar 22.
2010
요약 표시
This study was aimed to establish embryonic stem (ES)-like cells from blastocysts derived from somatic cell nuclear transfer (SCNT) in pig. Somatic cells isolated from both day-30 fetus and neonatal cloned piglet were used for donor cells. A total of 60 blastocysts (46 and 14 derived from fetal and neonatal fibroblast donor cells, respectively) were seeded onto a mitotically inactive mouse embryonic fibroblast (MEF) monolayer and two ES-like cell lines, one from each donor cell type, were established. They remained undifferentiated over more than 52 (fetal fibroblast-derived) and 48 (neonatal fibroblast-derived) passages, while retaining alkaline phosphatase activity and reactivity with ES specific markers Oct-4, stage-specific embryonic antigen-1 (SSEA-1), SSEA-4, TRA-1-60 and TRA-1-81. These ES-like cells maintained normal diploid karyotype throughout subculture and successfully differentiated into embryoid bodies that expressed three germ layer-specific genes (ectoderm: beta-III tubulin; endoderm: amylase; and mesoderm: enolase) after culture in leukemia inhibitory factor-free medium. Microsatellite analysis confirmed that they were genetically identical to its donor cells. Combined with gene targeting, our results may contribute to developing an efficient method for producing transgenic pigs for various purposes. | 20307349
 |
Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb.
Feng, Bo, et al.
Nat. Cell Biol., 11: 197-203 (2009)
2009
요약 표시
The dominant effect of transcription factors in imparting expanded potency is best exemplified by the reprogramming of fibroblasts to pluripotent cells using retrovirus-mediated transduction of defined transcription factors. In the murine system, Oct4, Sox2, c-Myc and Klf4 are sufficient to convert fibroblasts to induced pluripotent stem (iPS) cells that have many characteristics of embryonic stem (ES) cells. Here we show that the orphan nuclear receptor Esrrb functions in conjunction with Oct4 and Sox2 to mediate reprogramming of mouse embryonic fibroblasts (MEFs) to iPS cells. Esrrb-reprogrammed cells share similar expression and epigenetic signatures as ES cells. These cells are also pluripotent and can differentiate in vitro and in vivo into the three major embryonic cell lineages. Furthermore, these cells contribute to mouse chimaeras and are germline transmissible. In ES cells, Esrrb targets many genes involved in self-renewal and pluripotency. This suggests that Esrrb may mediate reprogramming through the upregulation of ES-cell-specific genes. Our findings also indicate that it is possible to reprogram MEFs without exogenous Klf transcription factors and link a nuclear receptor to somatic cell reprogramming. | 19136965
 |