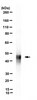
Activity-dependent anchoring of importin alpha at the synapse involves regulated binding to the cytoplasmic tail of the NR1-1a subunit of the NMDA receptor.
Jeffrey, Rachel A, et al.
J. Neurosci., 29: 15613-20 (2009)
2009
요약 표시
Synaptic plasticity, the capacity of neurons to change the strength of their connections with experience, provides a mechanism for learning and memory in the brain. Long-term plasticity requires new transcription, indicating that synaptically generated signals must be transported to the nucleus. Previous studies have described a role for importin nuclear transport adaptors in mediating the retrograde transport of signals from synapse to nucleus during plasticity. Here, we investigated the possibility that stimulus-induced translocation of importins from synapse to nucleus involves activity-dependent anchoring of importins at the synapse. We show that importin alpha binds to a nuclear localization signal (NLS) present in the cytoplasmic tail of NR1-1a. This interaction is disrupted by activation of NMDA receptors in cultured neurons and by stimuli that trigger late-phase, but not early-phase, long-term potentiation of CA3-CA1 synapses in acute hippocampal slices. In vitro PKC phosphorylation of GST-NR1-1a abolishes its ability to bind importin alpha in brain lysates, and the interaction of importin alpha and NR1 in neurons is modulated by PKC activity. Together, our results indicate that importin alpha is tethered at the postsynaptic density by binding to the NLS present in NR1-1a. This interaction is activity dependent, with importin alpha being released following NMDA receptor activation and phosphorylation rendering it available to bind soluble cargoes and transport them to the nucleus during transcription-dependent forms of neuronal plasticity. | 20016075
 |