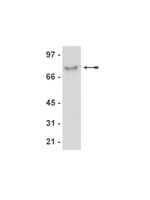
Genome-wide mapping of human DNA-replication origins: levels of transcription at ORC1 sites regulate origin selection and replication timing.
Dellino, GI; Cittaro, D; Piccioni, R; Luzi, L; Banfi, S; Segalla, S; Cesaroni, M; Mendoza-Maldonado, R; Giacca, M; Pelicci, PG
Genome research
23
1-11
2013
요약 표시
We report the genome-wide mapping of ORC1 binding sites in mammals, by chromatin immunoprecipitation and parallel sequencing (ChIP-seq). ORC1 binding sites in HeLa cells were validated as active DNA replication origins (ORIs) using Repli-seq, a method that allows identification of ORI-containing regions by parallel sequencing of temporally ordered replicating DNA. ORC1 sites were universally associated with transcription start sites (TSSs) of coding or noncoding RNAs (ncRNAs). Transcription levels at the ORC1 sites directly correlated with replication timing, suggesting the existence of two classes of ORIs: those associated with moderate/high transcription levels (≥1 RNA copy/cell), firing in early S and mapping to the TSSs of coding RNAs; and those associated with low transcription levels (less than 1 RNA copy/cell), firing throughout the entire S and mapping to TSSs of ncRNAs. These findings are compatible with a scenario whereby TSS expression levels influence the efficiency of ORC1 recruitment at G(1) and the probability of firing during S. | Western Blotting | 23187890
 |
RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly.
Huen, Michael S Y, et al.
Cell, 131: 901-14 (2007)
2007
요약 표시
DNA-damage signaling utilizes a multitude of posttranslational modifiers as molecular switches to regulate cell-cycle checkpoints, DNA repair, cellular senescence, and apoptosis. Here we show that RNF8, a FHA/RING domain-containing protein, plays a critical role in the early DNA-damage response. We have solved the X-ray crystal structure of the FHA domain structure at 1.35 A. We have shown that RNF8 facilitates the accumulation of checkpoint mediator proteins BRCA1 and 53BP1 to the damaged chromatin, on one hand through the phospho-dependent FHA domain-mediated binding of RNF8 to MDC1, on the other hand via its role in ubiquitylating H2AX and possibly other substrates at damage sites. Moreover, RNF8-depleted cells displayed a defective G2/M checkpoint and increased IR sensitivity. Together, our study implicates RNF8 as a novel DNA-damage-responsive protein that integrates protein phosphorylation and ubiquitylation signaling and plays a critical role in the cellular response to genotoxic stress. | | 18001825
 |