Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin.
Mallon, BS; Hamilton, RS; Kozhich, OA; Johnson, KR; Fann, YC; Rao, MS; Robey, PG
Stem cell research
12
376-86
2014
요약 표시
Many studies have compared the genetic and epigenetic profiles of human induced pluripotent stem cells (hiPSCs) to human embryonic stem cells (hESCs) and yet the picture remains unclear. To address this, we derived a population of neural precursor cells (NPCs) from the H1 (WA01) hESC line and generated isogenic iPSC lines by reprogramming. The gene expression and methylation profile of three lines were compared to the parental line and intermediate NPC population. We found no gene probe with expression that differed significantly between hESC and iPSC samples under undifferentiated or differentiated conditions. Analysis of the global methylation pattern also showed no significant difference between the two PSC populations. Both undifferentiated populations were distinctly different from the intermediate NPC population in both gene expression and methylation profiles. One point to note is that H1 is a male line and so extrapolation to female lines should be cautioned. However, these data confirm our previous findings that there are no significant differences between hESCs and hiPSCs at the gene expression or methylation level. | | 24374290
 |
Hyperoxia-induced hypertrophy and ion channel remodeling in left ventricle.
Panguluri, SK; Tur, J; Fukumoto, J; Deng, W; Sneed, KB; Kolliputi, N; Bennett, ES; Tipparaju, SM
American journal of physiology. Heart and circulatory physiology
304
H1651-61
2013
요약 표시
Ventricular arrhythmias account for high mortality in cardiopulmonary patients in intensive care units. Cardiovascular alterations and molecular-level changes in response to the commonly used oxygen treatment remains unknown. In the present study we investigated cardiac hypertrophy and cardiac complications in mice subjected to hyperoxia. Results demonstrate that there is a significant increase in average heart weight to tibia length (22%) in mice subjected to hyperoxia treatment vs. normoxia. Functional assessment was performed in mice subjected to hyperoxic treatment, and results demonstrate impaired cardiac function with decreased cardiac output and heart rate. Staining of transverse cardiac sections clearly demonstrates an increase in the cross-sectional area from hyperoxic hearts compared with control hearts. Quantitative real-time RT-PCR and Western blot analysis indicated differential mRNA and protein expression levels between hyperoxia-treated and control left ventricles for ion channels including Kv4.2 (-2 ± 0.08), Kv2.1 (2.54 ± 0.48), and Scn5a (1.4 ± 0.07); chaperone KChIP2 (-1.7 ± 0.06); transcriptional factors such as GATA4 (-1.5 ± 0.05), Irx5 (5.6 ± 1.74), NFκB1 (4.17 ± 0.43); hypertrophy markers including MHC-6 (2.17 ± 0.36) and MHC-7 (4.62 ± 0.76); gap junction protein Gja1 (4.4 ± 0.8); and microRNA processing enzyme Drosha (4.6 ± 0.58). Taken together, the data presented here clearly indicate that hyperoxia induces left ventricular remodeling and hypertrophy and alters the expression of Kv4.2 and MHC6/7 in the heart. | Western Blotting | 23585127
 |
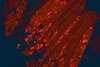
Histochemistry of ventricular heavy-chain myosins in cardiomyopathic Syrian hamsters treated with D-600.
Jasmin, G, et al.
Proc. Soc. Exp. Biol. Med., 188: 142-8 (1988)
1988
요약 표시
Monoclonal antibodies (MAb) have been used to study the distribution of ventricular heavy-chain (HC) myosins in cardiomyopathic UM-X7.1 Syrian hamsters. The Ab were identified as alpha and beta anti-HC myosins because of their ability to cross-react with ventricular V1 and V3 myosins, respectively. Cryostat frozen sections from the midventricle region of normal and myopathic hearts were processed for demonstration of these isomyosins by indirect immunofluorescence. In myopathic hearts, there was a shift of predominant alpha myosin toward the beta isoform with the time course of the hamster cardiomyopathy. A D-600 treatment while preventing cardiac necrotic lesions had little or no effect on the beta isomyosin conversion. It is inferred that the isomyosin shift during the progression of the hamster cardiomyopathy is unrelated to the necrotizing process and merely reflects the hypokynetism of the cardiomyocytes. | | 3287384
 |
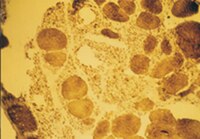
Local diversity of myosin expression in mammalian atrial muscles. Variations depending on age and thyroid state in the rat and the rabbit.
Dechesne, C A, et al.
Circ. Res., 57: 767-75 (1985)
1985
요약 표시
Rat, rabbit, pig, and bovine atrial myocardia were investigated with anti-alpha and anti-beta myosin heavy chain monoclonal antibodies. Analysis of atrial fibers by indirect immunofluorescence and assay of myosin heavy chains in tissue micro samples by immunoaffinity chromatography revealed both heterogeneity and plasticity in the atrial myosin heavy chains, undetected by electrophoresis of native atrial myosins under nondenaturing conditions. We found both alpha- and beta-like myosin heavy chains to be expressed in rat and rabbit, as they are in pig and bovine, atrial myocardia. They were regionally distributed within atrial muscles. The beta-like myosin heavy chains were present at much lower levels in rat and rabbit atria than in pig and bovine atria. Young rat atrial myosin was composed of only alpha-like heavy chains. In the rat and the rabbit, hyperthyroidism induced a beta- to alpha-like myosin heavy chain transition, which was considerable in the right atria and complete in the left atria. In the rat, thyroidectomy induced a moderate alpha- to beta-like myosin heavy chain transition, visible in the left atria. The significance of this atrial myosin heavy chain polymorphism is discussed in relation to the existence of anatomical localizations of the two myosin variants. | | 3902278
 |