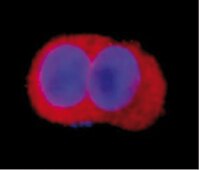
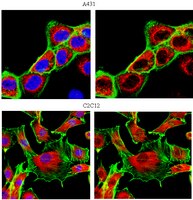
Tyrosine phosphorylation profiling via in situ proximity ligation assay.
Elfineh, L; Classon, C; Asplund, A; Pettersson, U; Kamali-Moghaddam, M; Lind, SB
BMC cancer
14
435
2014
요약 표시
Tyrosine phosphorylation (pTyr) is an important cancer relevant posttranslational modification since it regulates protein activity and cellular localization. By controlling cell growth and differentiation it plays an important role in tumor development. This paper describes a novel approach for detection and visualization of a panel of pTyr proteins in tumors using in situ proximity ligation assay.K562 leukemia cells were treated with tyrosine kinase and/or phosphatase inhibitors to induce differences in pTyr levels and mimic cells with different malignant properties. Cells were then probed with one antibody against the pTyr modification and another probe against the detected protein, resulting in a detectable fluorescent signal once the probes were in proximity.Total and protein specific pTyr levels on ABL, SHC, ERK2 and PI3K proteins were detected and samples of control and treated cells were distinguished at the pTyr level using this novel approach. Promising results were also detected for formalin fixed and paraffin embedded cells in the micro array format.This application of in situ proximity ligation assay is valuable in order to study the pTyr modification of a panel of proteins in large data sets to validate mass spectrometric data and to be combined with tissue microarrays. The approach offers new opportunities to reveal the pTyr signatures in cells of different malignant properties that can be used as biomarker of disease in the future. | | 24928687
 |
Granulosa cell expression of G1/S phase cyclins and cyclin-dependent kinases in PMSG-induced follicle growth.
Jennifer D Cannon,Mary Cherian-Shaw,Tara Lovekamp-Swan,Charles L Chaffin
Molecular and cellular endocrinology
264
2007
요약 표시
Follicular development involves a complex orchestration of granulosa cell proliferation and differentiation. It is becoming increasingly apparent that the rate of granulosa cell proliferation declines as follicles reach the large antral status, prior to an ovulatory gonadotropin stimulus, although a precise time course and mechanism for this decline has not been described. The goal of the present study was to characterize granulosa cell proliferation following the onset of antral follicle growth in PMSG-primed immature rats, with emphasis on G1/S phase cyclins and cyclin-dependent kinases. Flow cytometric analysis demonstrated that the percentage of granulosa cells in S phase peaked 24-30 h post-PMSG and declined to control levels 48 h after PMSG administration. Expression of both Cyclin D2 and Cdk 4 was highest 12h post-PMSG and decreased to control levels by 48 h. In addition, Cdk 2 protein increased transiently 12-24h after PMSG. Cyclin E expression increased significantly by 12h but remained elevated through 48 h, and multiple isoforms of Cyclin E were observed with increased proliferation. Both Cdk 4 and Cdk 2 activity parallel protein expression, although, changes in Cdk 2 were more marked. Levels of mRNA for the cell cycle inhibitors p21CIP1 and p27KIP1 increased significantly by 48 h post-PMSG. These results demonstrate that PMSG-stimulated movement of granulosa cells across the G1/S boundary during follicle growth is transient. In addition, the control of granulosa cell proliferation may reside through the regulation of both Cdk 2 and Cdk 4. | | 17084963
 |
Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor Rapamycin.
Molhoek, KR; Brautigan, DL; Slingluff, CL
Journal of translational medicine
3
39
2005
요약 표시
Targeted inhibition of protein kinases is now acknowledged as an effective approach for cancer therapy. However, targeted therapies probably have limited success because cancer cells have alternate pathways for survival and proliferation thereby avoiding inhibition. We tested the hypothesis that combination of targeted agents would be more effective than single agents in arresting melanoma cell proliferation.We evaluated whether BAY43-9006, an inhibitor of the B-Raf kinase, and rapamycin, an inhibitor of the mTOR kinase, would inhibit serum-stimulated proliferation of human melanoma cell lines, either alone or in combination. Proliferation was measured by quantitating melanoma cell numbers with a luciferase for ATP. Phosphorylation of proteins downstream of targeted kinase(s) was assayed by immunoblots. Statistical significance was determined with the Student-T test. Isobologram analysis was performed to distinguish additive versus synergistic effects of combinations of drugs.Serum-stimulated proliferation of multiple human melanoma cell lines was inhibited by BAY43-9006 and by rapamycin. Melanoma cells containing the B-Raf mutation V599E were more sensitive than cells with wild-type B-raf to 10 nM doses of both BAY43-9006 and rapamycin. Regardless of B-Raf mutational status, the combination of low dose rapamycin and BAY43-9006 synergistically inhibited melanoma cell proliferation. As expected, rapamycin inhibited the phosphorylation of mTOR substrates, p70S6K and 4EBP1, and BAY43-9006 inhibited phosphorylation of ERK, which is dependent on B-Raf activity. We also observed unexpected rapamycin inhibition of the phosphorylation of ERK, as well as BAY43-9006 inhibition of the phosphorylation of mTOR substrates, p70S6K and 4EBP1.There was synergistic inhibition of melanoma cell proliferation by the combination of rapamycin and BAY 43-9006, and unexpected inhibition of two signaling pathways by agents thought to target only one of those pathways. These results indicate that combinations of inhibitors of mTOR and of the B-raf signaling pathways may be more effective as a treatment for melanoma than use of either agent alone. 기사 전문 | | 16255777
 |
The beta3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism
Soeder, K. J., et al
J Biol Chem, 274:12017-22 (1999)
1999
| Immunoblotting (Western) | 10207024
 |
Distinct mechanisms of activation of Stat1 and Stat3 by platelet-derived growth factor receptor in a cell-free system.
Vignais, M L and Gilman, M
Mol. Cell. Biol., 19: 3727-35 (1999)
1999
요약 표시
Ligand-dependent activation of the platelet-derived growth factor receptor (PDGFR) in fibroblasts in culture leads to the activation of the JAK family of protein-tyrosine kinases and of the transcription factors Stat1 and Stat3. To determine the biochemical mechanism of STAT activation by PDGFR, we devised a cell-free system composed of a membrane fraction from cells overexpressing PDGFR. When supplemented with crude cytosol, the membrane fraction supported PDGF- and ATP-dependent activation of both Stat1 and Stat3. However, the extent of Stat3 activation differed depending on the source of the cytosolic fraction. Using purified recombinant STAT proteins produced in Escherichia coli, we found that Stat1 could be activated by immunopurified PDGFR and showed no additional requirement for membrane- or cytosol-derived proteins. In contrast, activation of Stat3 exhibited a strong requirement for the cytosolic fraction. The activity present in the cytosolic fraction could be depleted with antibodies to JAK proteins. We conclude that the mechanisms of activation of Stat1 and Stat3 by PDGFR are distinct. Stat1 activation appears to result from a direct interaction with the receptor, whereas Stat3 activation additionally requires JAK proteins. | Immunoprecipitation | 10207096
 |
The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils
Kiefer, F., et al
Mol Cell Biol, 18:4209-20 (1998)
1998
| Immunoblotting (Western) | 9632805
 |
Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38.
Yuasa, T, et al.
J. Biol. Chem., 273: 22681-92 (1998)
1998
요약 표시
Tumor necrosis factor (TNF) elicits a diverse array of inflammatory responses through engagement of its type-1 receptor (TNFR1). Many of these responses require de novo gene expression mediated by the activator protein-1 (AP-1) transcription factor. We investigated the mechanism by which TNFR1 recruits the stress-activated protein kinases (SAPKs) and the p38s, two mitogen-activated protein kinase (MAPK) families that together regulate AP-1. We show that the human SPS1 homologue germinal center kinase (GCK) can interact in vivo with the TNFR1 signal transducer TNFR-associated factor-2 (TRAF2) and with MAPK/ERK kinase kinase 1 (MEKK1), a MAPK kinase kinase (MAPKKK) upstream of the SAPKs, thereby coupling TRAF2 to the SAPKs. Receptor interacting protein (RIP) is a second TNFR signal transducer which can bind TRAF2. We show that RIP activates both p38 and SAPK; and that TRAF2 activation of p38 requires RIP. We also demonstrate that the RIP noncatalytic intermediate domain associates in vivo with an endogenous MAPKKK that can activate the p38 pathway in vitro. Thus, TRAF2 initiates SAPK and p38 activation by binding two proximal protein kinases: GCK and RIP. GCK and RIP, in turn, signal by binding MAPKKKs upstream of the SAPKs and p38s. | Immunoblotting (Western) | 9712898
 |
Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB
Kauffmann-Zeh, A., et al
Nature, 385:544-8 (1997)
1997
| Immunoblotting (Western) | 9020362
 |
An incomplete program of cellular tyrosine phosphorylations induced by kinase-defective epidermal growth factor receptors
Wright, J. D., et al
J Biol Chem, 270:12085-93 (1995)
1995
| IP-Kinase Assay | 7538132
 |
The serine/threonine phosphatase inhibitor, calyculin A, inhibits and dissociates macrophage responses to lipopolysaccharide.
Barber, S A, et al.
J. Immunol., 155: 1404-10 (1995)
1995
요약 표시
LPS-stimulated macrophages (M phi) produce inflammatory mediators that are largely responsible for the pathophysiology associated with septic shock. M phi respond to LPS with rapid protein phosphorylation and dephosphorylation on serine, threonine, and tyrosine residues. If these events are critical for the cellular response to LPS, the kinases and/or phosphatases involved may be vulnerable targets for pharmacologic intervention. Recent studies demonstrated that tyrosine kinase inhibitors block LPS-induced tyrosine phosphorylation of MAP kinases as well as TNF-alpha and IL-1 beta production. To investigate a role for serine/threonine phosphatases, we evaluated the effect of calyculin A, a potent serine/threonine phosphatase inhibitor, on LPS stimulation of murine M phi. Pretreatment of M phi with calyculin A inhibited LPS-induced expression of six immediate-early genes: TNF-alpha, IL-1 beta, IFN-beta, IP-10, IRF-1, and TNFR-2. Calyculin A added 1.5 h after LPS treatment greatly reduced accumulation of IP-10, IRF-1, and TNFR-2 mRNA, but not TNF-alpha, IL-1 beta, and IFN-beta mRNA. Calyculin A, in the absence or presence of LPS, resulted in sustained tyrosine phosphorylation of the MAP kinases. These findings suggest that an "early" serine/threonine phosphatase activity is essential for LPS stimulation of M phi and that the activation of MAP kinases is not sufficient for the induction of these immediate-early genes. The requirement for a "late" phosphatase activity for expression of a subset of LPS-inducible genes dissociates at least two regulatory pathways in LPS signal transduction. | Immunoblotting (Western) | 7636205
 |