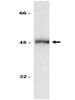
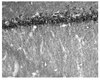
Biological Effects Induced by Specific Advanced Glycation End Products in the Reconstructed Skin Model of Aging.
Pageon, H; Zucchi, H; Dai, Z; Sell, DR; Strauch, CM; Monnier, VM; Asselineau, D
BioResearch open access
4
54-64
2015
요약 표시
Advanced glycation end products (AGEs) accumulate in the aging skin. To understand the biological effects of individual AGEs, skin reconstructed with collagen selectively enriched with N(ɛ)-(carboxymethyl)-lysine (CML), N(ɛ)-(carboxyethyl)-lysine (CEL), methylglyoxal hydroimidazolone (MG-H1), or pentosidine was studied. Immunohistochemistry revealed increased expression of α6 integrin at the dermal epidermal junction by CEL and CML (pless than 0.01). Laminin 5 was diminished by CEL and MG-H1 (pless than 0.05). Both CML and CEL induced a robust increase (pless than 0.01) in procollagen I. In the culture medium, IL-6, VEGF, and MMP1 secretion were significantly decreased (pless than 0.05) by MG-H1. While both CEL and CML decreased MMP3, only CEL decreased IL-6 and TIMP1, while CML stimulated TIMP1 synthesis significantly (pless than 0.05). mRNA expression studies using qPCR in the epidermis layer showed that CEL increased type 7 collagen (COL7A1), β1, and α6 integrin, while CML increased only COL7A1 (pless than 0.05). MG-H1-modified collagen had no effect. Importantly, in the dermis layer, MMP3 mRNA expression was increased by both CML and MG-H1. CML also significantly increased the mRNAs of MMP1, TIMP1, keratinocyte growth factor (KGF), IL-6, and monocyte chemoattractant protein 1 (MCP1) (pless than 0.05). Mixed effects were present in CEL-rich matrix. Minimally glycoxidized pentosidine-rich collagen suppressed most mRNAs of the genes studied (pless than 0.05) and decreased VEGF and increased MCP1 protein expression. Taken together, this model of the aging skin suggests that a combination of AGEs tends to counterbalance and thus minimizes the detrimental biological effects of individual AGEs. | | | 26309782
 |
Lysyl Hydroxylase 3 Localizes to Epidermal Basement Membrane and Is Reduced in Patients with Recessive Dystrophic Epidermolysis Bullosa.
Watt, SA; Dayal, JH; Wright, S; Riddle, M; Pourreyron, C; McMillan, JR; Kimble, RM; Prisco, M; Gartner, U; Warbrick, E; McLean, WH; Leigh, IM; McGrath, JA; Salas-Alanis, JC; Tolar, J; South, AP
PloS one
10
e0137639
2015
요약 표시
Recessive dystrophic epidermolysis bullosa (RDEB) is caused by mutations in COL7A1 resulting in reduced or absent type VII collagen, aberrant anchoring fibril formation and subsequent dermal-epidermal fragility. Here, we identify a significant decrease in PLOD3 expression and its encoded protein, the collagen modifying enzyme lysyl hydroxylase 3 (LH3), in RDEB. We show abundant LH3 localising to the basement membrane in normal skin which is severely depleted in RDEB patient skin. We demonstrate expression is in-part regulated by endogenous type VII collagen and that, in agreement with previous studies, even small reductions in LH3 expression lead to significantly less secreted LH3 protein. Exogenous type VII collagen did not alter LH3 expression in cultured RDEB keratinocytes and we show that RDEB patients receiving bone marrow transplantation who demonstrate significant increase in type VII collagen do not show increased levels of LH3 at the basement membrane. Our data report a direct link between LH3 and endogenous type VII collagen expression concluding that reduction of LH3 at the basement membrane in patients with RDEB will likely have significant implications for disease progression and therapeutic intervention. | | | 26380979
 |
Type VII collagen regulates expression of OATP1B3, promotes front-to-rear polarity and increases structural organisation in 3D spheroid cultures of RDEB tumour keratinocytes.
Dayal, JH; Cole, CL; Pourreyron, C; Watt, SA; Lim, YZ; Salas-Alanis, JC; Murrell, DF; McGrath, JA; Stieger, B; Jahoda, C; Leigh, IM; South, AP
Journal of cell science
127
740-51
2014
요약 표시
Type VII collagen is the main component of anchoring fibrils, structures that are integral to basement membrane homeostasis in skin. Mutations in the gene encoding type VII collagen COL7A1 cause recessive dystrophic epidermolysis bullosa (RDEB) an inherited skin blistering condition complicated by frequent aggressive cutaneous squamous cell carcinoma (cSCC). OATP1B3, which is encoded by the gene SLCO1B3, is a member of the OATP (organic anion transporting polypeptide) superfamily responsible for transporting a wide range of endogenous and xenobiotic compounds. OATP1B3 expression is limited to the liver in healthy tissues, but is frequently detected in multiple cancer types and is reported to be associated with differing clinical outcome. The mechanism and functional significance of tumour-specific expression of OATP1B3 has yet to be determined. Here, we identify SLCO1B3 expression in tumour keratinocytes isolated from RDEB and UV-induced cSCC and demonstrate that SLCO1B3 expression and promoter activity are modulated by type VII collagen. We show that reduction of SLCO1B3 expression upon expression of full-length type VII collagen in RDEB cSCC coincides with acquisition of front-to-rear polarity and increased organisation of 3D spheroid cultures. In addition, we show that type VII collagen positively regulates the abundance of markers implicated in cellular polarity, namely ELMO2, PAR3, E-cadherin, B-catenin, ITGA6 and Ln332. | | | 24357722
 |
A simple alkaline method for decellularizing human amniotic membrane for cell culture.
Saghizadeh, M; Winkler, MA; Kramerov, AA; Hemmati, DM; Ghiam, CA; Dimitrijevich, SD; Sareen, D; Ornelas, L; Ghiasi, H; Brunken, WJ; Maguen, E; Rabinowitz, YS; Svendsen, CN; Jirsova, K; Ljubimov, AV
PloS one
8
e79632
2013
요약 표시
Human amniotic membrane is a standard substratum used to culture limbal epithelial stem cells for transplantation to patients with limbal stem cell deficiency. Various methods were developed to decellularize amniotic membrane, because denuded membrane is poorly immunogenic and better supports repopulation by dissociated limbal epithelial cells. Amniotic membrane denuding usually involves treatment with EDTA and/or proteolytic enzymes; in many cases additional mechanical scraping is required. Although ensuring limbal cell proliferation, these methods are not standardized, require relatively long treatment times and can result in membrane damage. We propose to use 0.5 M NaOH to reliably remove amniotic cells from the membrane. This method was used before to lyse cells for DNA isolation and radioactivity counting. Gently rubbing a cotton swab soaked in NaOH over the epithelial side of amniotic membrane leads to nearly complete and easy removal of adherent cells in less than a minute. The denuded membrane is subsequently washed in a neutral buffer. Cell removal was more thorough and uniform than with EDTA, or EDTA plus mechanical scraping with an electric toothbrush, or n-heptanol plus EDTA treatment. NaOH-denuded amniotic membrane did not show any perforations compared with mechanical or thermolysin denuding, and showed excellent preservation of immunoreactivity for major basement membrane components including laminin α2, γ1-γ3 chains, α1/α2 and α6 type IV collagen chains, fibronectin, nidogen-2, and perlecan. Sodium hydroxide treatment was efficient with fresh or cryopreserved (10% dimethyl sulfoxide or 50% glycerol) amniotic membrane. The latter method is a common way of membrane storage for subsequent grafting in the European Union. NaOH-denuded amniotic membrane supported growth of human limbal epithelial cells, immortalized corneal epithelial cells, and induced pluripotent stem cells. This simple, fast and reliable method can be used to standardize decellularized amniotic membrane preparations for expansion of limbal stem cells in vitro before transplantation to patients. | | | 24236148
 |
Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma.
Kinoshita, T; Nohata, N; Hanazawa, T; Kikkawa, N; Yamamoto, N; Yoshino, H; Itesako, T; Enokida, H; Nakagawa, M; Okamoto, Y; Seki, N
British journal of cancer
109
2636-45
2013
요약 표시
Our recent studies of microRNA (miRNA) expression signatures demonstrated that microRNA-29s (miR-29s; miR-29a/b/c) were significantly downregulated in head and neck squamous cell carcinoma (HNSCC) and were putative tumour-suppressive miRNAs in human cancers. Our aim in this study was to investigate the functional significance of miR-29s in cancer cells and to identify novel miR-29s-mediated cancer pathways and responsible genes in HNSCC oncogenesis and metastasis.Gain-of-function studies using mature miR-29s were performed to investigate cell proliferation, migration and invasion in two HNSCC cell lines (SAS and FaDu). To identify miR-29s-mediated molecular pathways and targets, we utilised gene expression analysis and in silico database analysis. Loss-of-function assays were performed to investigate the functional significance of miR-29s target genes.Restoration of miR-29s in SAS and FaDu cell lines revealed significant inhibition of cancer cell migration and invasion. Gene expression data and in silico analysis demonstrated that miR-29s modulated the focal adhesion pathway. Moreover, laminin γ2 (LAMC2) and α6 integrin (ITGA6) genes were candidate targets of the regulation of miR-29s. Luciferase reporter assays showed that miR-29s directly regulated LAMC2 and ITGA6. Silencing of LAMC2 and ITGA6 genes significantly inhibited cell migration and invasion in cancer cells.Downregulation of miR-29s was a frequent event in HNSCC. The miR-29s acted as tumour suppressors and directly targeted laminin-integrin signalling. Recognition of tumour-suppressive miRNA-mediated cancer pathways provides new insights into the potential mechanisms of HNSCC oncogenesis and metastasis and suggests novel therapeutic strategies for the disease. | | | 24091622
 |
IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway.
Chen, N; Debnath, J
Autophagy
9
1214-27
2013
요약 표시
Adherent cells require proper integrin-mediated extracellular matrix (ECM) engagement for growth and survival; normal cells deprived of proper ECM contact undergo anoikis. At the same time, autophagy is induced as a survival pathway in both fibroblasts and epithelial cells upon ECM detachment. Here, we further define the intracellular signals that mediate detachment-induced autophagy and uncover an important role for the IκB kinase (IKK) complex in the induction of autophagy in mammary epithelial cells (MECs) deprived of ECM contact. Whereas the PI3K-AKT-MTORC1 pathway activation potently inhibits autophagy in ECM-detached fibroblasts, enforced activation of this pathway is not sufficient to suppress detachment-induced autophagy in MECs. Instead, inhibition of IKK, as well as its upstream regulator, MAP3K7/TAK1, significantly attenuates detachment-induced autophagy in MECs. Furthermore, function-blocking experiments corroborate that both IKK activation and autophagy induction result from decreased ITGA3-ITGB1 (α3β1 integrin) function. Finally, we demonstrate that pharmacological IKK inhibition enhances anoikis and accelerates luminal apoptosis during acinar morphogenesis in three-dimensional culture. Based on these results, we propose that the IKK complex functions as a key mediator of detachment-induced autophagy and anoikis resistance in epithelial cells. | | | 23778976
 |
p63 attenuates epithelial to mesenchymal potential in an experimental prostate cell model.
Olsen, JR; Oyan, AM; Rostad, K; Hellem, MR; Liu, J; Li, L; Micklem, DR; Haugen, H; Lorens, JB; Rotter, V; Ke, XS; Lin, B; Kalland, KH
PloS one
8
e62547
2013
요약 표시
The transcription factor p63 is central for epithelial homeostasis and development. In our model of epithelial to mesenchymal transition (EMT) in human prostate cells, p63 was one of the most down-regulated transcription factors during EMT. We therefore investigated the role of p63 in EMT. Over-expression of the predominant epithelial isoform ΔNp63α in mesenchymal type cells of the model led to gain of several epithelial characteristics without resulting in a complete mesenchymal to epithelial transition (MET). This was corroborated by a reciprocal effect when p63 was knocked down in epithelial EP156T cells. Global gene expression analyses showed that ΔNp63α induced gene modules involved in both cell-to-cell and cell-to-extracellular-matrix junctions in mesenchymal type cells. Genome-wide analysis of p63 binding sites using ChIP-seq analyses confirmed binding of p63 to regulatory areas of genes associated with cell adhesion in prostate epithelial cells. DH1 and ZEB1 are two elemental factors in the control of EMT. Over-expression and knock-down of these factors, respectively, were not sufficient alone or in combination with ΔNp63α to reverse completely the mesenchymal phenotype. The partial reversion of epithelial to mesenchymal transition might reflect the ability of ΔNp63α, as a key co-ordinator of several epithelial gene expression modules, to reduce epithelial to mesenchymal plasticity (EMP). The utility of ΔNp63α expression and the potential of reduced EMP in order to counteract metastasis warrant further investigation. | | | 23658742
 |
The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens.
Behrens, DT; Villone, D; Koch, M; Brunner, G; Sorokin, L; Robenek, H; Bruckner-Tuderman, L; Bruckner, P; Hansen, U
The Journal of biological chemistry
287
18700-9
2012
요약 표시
The basement membrane between the epidermis and the dermis is indispensable for normal skin functions. It connects, and functionally separates, the epidermis and the dermis. To understand the suprastructural and functional basis of these connections, heterotypic supramolecular aggregates were isolated from the dermal-epidermal junction zone of human skin. Individual suprastructures were separated and purified by immunomagnetic beads, each recognizing a specific, molecular component of the aggregates. The molecular compositions of the suprastructures were determined by immunogold electron microscopy and immunoblotting. A composite of two networks was obtained from fibril-free suspensions by immunobeads recognizing either laminin 332 or collagen IV. After removal of perlecan-containing suprastructures or after enzyme digestion of heparan sulfate chains, a distinct network with a diffuse electron-optical appearance was isolated with magnetic beads coated with antibodies to collagen IV. The second network was more finely grained and comprised laminin 332 and laminins with α5-chains. The core protein of perlecan was an exclusive component of this network whereas its heparan sulfate chains were integrated into the collagen IV-containing network. Nidogens 1 and 2 occurred in both networks but did not form strong molecular cross-bridges. Their incorporation into one network appeared to be masked after their incorporation into the other one. We conclude that the epidermal basement membrane is a composite of two structurally independent networks that are tightly connected in a spot-welding-like manner by perlecan-containing aggregates. | | | 22493504
 |
Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma.
Kinoshita, T; Hanazawa, T; Nohata, N; Kikkawa, N; Enokida, H; Yoshino, H; Yamasaki, T; Hidaka, H; Nakagawa, M; Okamoto, Y; Seki, N
Oncotarget
3
1386-400
2012
요약 표시
Recent our microRNA (miRNA) expression signature revealed that expression of microRNA-218 (miR-218) was reduced in cancer tissues, suggesting a candidate of tumor suppressor in head and neck squamous cell carcinoma (HNSCC). The aim of this study was to investigate the functional significance of miR-218 and its mediated moleculer pathways in HNSCC. Restoration of miR-218 in cancer cells led to significant inhibition of cell migration and invasion activities in HNSCC cell lines (FaDu and SAS). Genome-wide gene expression analysis of miR-218 transfectants and in silico database analysis showed that focal adhesion pathway was a promising candidate of miR-218 target pathways. The laminins are an important and biologically active part of the basal lamina, the function of that are various such as influencing cell differentiation, migration and adhesion as well as proliferation and cell survival. Interestingly, all components of laminin-332 (LAMA3, LAMB3 and LAMC2) are listed on the candidate genes in focal adhesion pathway. Furthermore, we focused on LAMB3 which has a miR-218 target site and gene expression studies and luciferase reporter assays showed that LAMB3 was directly regulated by miR-218. Silencing study of LAMB3 demonstrated significant inhibition of cell migration and invasion. In clinical specimens with HNSCC, the expression levels of laminin-332 were significantly upregulated in cancer tissues compared to adjacent non-cancerous tissues. Our analysis data showed that tumor suppressive miR-218 contributes to cancer cell migration and invasion through regulating focal adhesion pathway, especially laminin-332. Tumor suppressive miRNA-mediated novel cancer pathways provide new insights into the potential mechanisms of HNSCC oncogenesis. | | | 23159910
 |
A metabolic prosurvival role for PML in breast cancer.
Carracedo, A; Weiss, D; Leliaert, AK; Bhasin, M; de Boer, VC; Laurent, G; Adams, AC; Sundvall, M; Song, SJ; Ito, K; Finley, LS; Egia, A; Libermann, T; Gerhart-Hines, Z; Puigserver, P; Haigis, MC; Maratos-Flier, E; Richardson, AL; Schafer, ZT; Pandolfi, PP
The Journal of clinical investigation
122
3088-100
2012
요약 표시
Cancer cells exhibit an aberrant metabolism that facilitates more efficient production of biomass and hence tumor growth and progression. However, the genetic cues modulating this metabolic switch remain largely undetermined. We identified a metabolic function for the promyelocytic leukemia (PML) gene, uncovering an unexpected role for this bona fide tumor suppressor in breast cancer cell survival. We found that PML acted as both a negative regulator of PPARγ coactivator 1A (PGC1A) acetylation and a potent activator of PPAR signaling and fatty acid oxidation. We further showed that PML promoted ATP production and inhibited anoikis. Importantly, PML expression allowed luminal filling in 3D basement membrane breast culture models, an effect that was reverted by the pharmacological inhibition of fatty acid oxidation. Additionally, immunohistochemical analysis of breast cancer biopsies revealed that PML was overexpressed in a subset of breast cancers and enriched in triple-negative cases. Indeed, PML expression in breast cancer correlated strikingly with reduced time to recurrence, a gene signature of poor prognosis, and activated PPAR signaling. These findings have important therapeutic implications, as PML and its key role in fatty acid oxidation metabolism are amenable to pharmacological suppression, a potential future mode of cancer prevention and treatment. | | | 22886304
 |