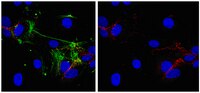
LFA-1 and ICAM-1 expression induced during melanoma-endothelial cell co-culture favors the transendothelial migration of melanoma cell lines in vitro.
Ghislin, S; Obino, D; Middendorp, S; Boggetto, N; Alcaide-Loridan, C; Deshayes, F
BMC cancer
12
455
2012
요약 표시
Patients with metastatic melanoma have a poor median rate of survival. It is therefore necessary to increase our knowledge about melanoma cell dissemination which includes extravasation, where cancer cells cross the endothelial barrier. Extravasation is well understood during travelling of white blood cells, and involves integrins such as LFA-1 (composed of two chains, CD11a and CD18) expressed by T cells, while ICAM-1 is induced during inflammation by endothelial cells. Although melanoma cell lines cross endothelial cell barriers, they do not express LFA-1. We therefore hypothesized that melanoma-endothelial cell co-culture might induce the LFA-1/ICAM ligand/receptor couple during melanoma transmigration.A transwell approach has been used as well as blocking antibodies against CD11a, CD18 and ICAM-1. Data were analyzed with an epifluorescence microscope. Fluorescence intensity was quantified with the ImageJ software.We show here that HUVEC-conditioned medium induce cell-surface expression of LFA-1 on melanoma cell lines. Similarly melanoma-conditioned medium activates ICAM-1 expression in endothelial cells. Accordingly blocking antibodies of ICAM-1, CD11a or CD18 strongly decrease melanoma transmigration. We therefore demonstrate that melanoma cells can cross endothelial monolayers in vitro due to the induction of ICAM-1 and LFA-1 occurring during the co-culture of melanoma and endothelial cells. Our data further suggest a role of LFA-1 and ICAM-1 in the formation of melanoma cell clumps enhancing tumor cell transmigration.Melanoma-endothelial cell co-culture induces LFA-1 and ICAM-1 expression, thereby favoring in vitro melanoma trans-migration. | 23039186
 |
Evaluation of the sclerotherapeutic efficacy of ethanol, polidocanol, and OK-432 using an in vitro model.
William Mol, Hiroshi Furukawa, Satoru Sasaki, Utano Tomaru, Toshihiko Hayashi, Akira Saito, Munetomo Nagao, Noriko Saito, Shinya Hata, Yuhei Yamamoto
Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]
33
1452-9
2007
요약 표시
BACKGROUND: Sclerosants are used to treat vascular malformations. Owing to variations in the flow, the injected concentrations and the duration of exposure of these sclerosants are altered. Therefore, the clinical effectiveness of sclerotherapy is variable. OBJECTIVE: The objective was to evaluate the differences in clinical response, usually observed among ethanol, polidocanol, and OK-432, using an in vitro sclerotherapy model. METHODS: Endothelial cells were cultured and exposed to different concentrations of the sclerosants for 5 seconds and the remaining viable cells were counted using a MTT assay kit. Dyes were used to visualize the morphologic changes. Precipitant formation in blood was also evaluated. Finally, the degree of ICAM-1 expression, after exposure to lower concentrations of these sclerosants, was studied using immunocytochemistry. RESULTS: Only ethanol causes precipitant formation and kills almost all cells from 30% concentration. Polidocanol begins to disrupt the cell membrane from 0.0125% onward. Only OK-432 induces ICAM-1 expression. CONCLUSION: Ethanol's strong precipitant-forming effect may induce thromboembolism, thus enhancing sclerosis. Polidocanol's endothelial cell-lysing effect was clearly documented. OK-432 may mediate its effect by inducing inflammatory response of the endothelium via ICAM-1 expression. This in vitro model may be useful in evaluating other sclerosants as well. | 18076610
 |
Roles of phosphatidylinositol 3-kinase and NF-kappaB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence.
Smith, MS; Bivins-Smith, ER; Tilley, AM; Bentz, GL; Chan, G; Minard, J; Yurochko, AD
Journal of virology
81
7683-94
2007
요약 표시
Infected peripheral blood monocytes are proposed to play a key role in the hematogenous dissemination of human cytomegalovirus (HCMV) to tissues, a critical step in the establishment of HCMV persistence and the development of HCMV-associated diseases. We recently provided evidence for a unique strategy involved in viral dissemination: HCMV infection of primary human monocytes promotes their transendothelial migration and differentiation into proinflammatory macrophages permissive for the replication of the original input virus. To decipher the mechanism of hematogenous spread, we focused on the viral dysregulation of early cellular processes involved in transendothelial migration. Here, we present evidence that both phosphatidylinositol 3-kinase [PI(3)K] and NF-kappaB activities were crucial for the HCMV induction of monocyte motility and firm adhesion to endothelial cells. We found that the beta(1) integrins, the beta(2) integrins, intracellular adhesion molecule 1 (ICAM-1), and ICAM-3 were upregulated following HCMV infection and that they played a key role in the firm adhesion of infected monocytes to the endothelium. The viral regulation of adhesion molecule expression is complex, with PI(3)K and NF-kappaB affecting the expression of each adhesion molecule at different stages of the expression cascade. Our data demonstrate key roles for PI(3)K and NF-kappaB signaling in the HCMV-induced cellular changes in monocytes and identify the biological rationale for the activation of these pathways in infected monocytes, which together suggest a mechanism for how HCMV promotes viral spread to and persistence within host organs. 기사 전문 | 17507481
 |
Lymphocyte function-associated antigen-1-mediated T cell adhesion is impaired by low molecular weight phosphotyrosine phosphatase-dependent inhibition of FAK activity.
Giannoni, E; Chiarugi, P; Cozzi, G; Magnelli, L; Taddei, ML; Fiaschi, T; Buricchi, F; Raugei, G; Ramponi, G
The Journal of biological chemistry
278
36763-76
2003
요약 표시
Protein tyrosine phosphorylation is one of the earliest signaling events detected in response to lymphocyte function-associated antigen-1 (LFA-1) engagement during lymphocyte adhesion. In particular, the focal adhesion kinase p125FAK, involved in the modulation and rearrangement of the actin cytoskeleton, seems to be a crucial mediator of LFA-1 signaling. Herein, we investigate the role of a FAK tyrosine phosphatase, namely low molecular weight phosphotyrosine phosphatase (LMW-PTP), in the modulation of LFA-1-mediated T cell adhesion. Overexpression of LMW-PTP in Jurkat cells revealed an impairment of LFA-1-dependent cell-cell adhesion upon T cell receptor (TCR) stimulation. Moreover, in these conditions LMW-PTP causes FAK dephosphorylation, thus preventing the activation of FAK downstream pathways. Our results also demonstrated that, upon antigen stimulation, LMW-PTP-dependent FAK inhibition is associated to a strong reduction of LFA-1 and TCR co-clustering toward a single region of T cell surface, thus causing an impairment of receptor activity by preventing changes in their avidity state. Because co-localization of both LFA-1 and TCR is an essential event during encounters of T cells with antigen-presenting cells and immunological synapse (IS) formation, we suggest an intriguing role of LMW-PTP in IS establishment and stabilization through the negative control of FAK activity and, in turn, of cell surface receptor redistribution. | 12815062
 |
Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1.
Irene M Pedersen, Shinichi Kitada, Lorenzo M Leoni, Juan M Zapata, James G Karras, Nobuhiro Tsukada, Thomas J Kipps, Yong Sung Choi, Frank Bennett, John C Reed
Blood
100
1795-801
2002
요약 표시
Chronic lymphocytic leukemia (CLL) B cells have defects in apoptosis pathways and therefore accumulate in vivo. However, when removed from the patient and cultured in vitro, these malignant cells rapidly undergo apoptosis. Recent studies suggest that leukemia cell survival is influenced by interactions with nonleukemia cells in the microenvironment of lymph nodes, marrow, and other tissues. To model such cell-cell interactions in vitro, we cultured freshly isolated CLL B cells with a follicular dendritic cell line, HK. CLL B cells cocultured with HK cells were protected from apoptosis, either spontaneous or induced by treatment with anticancer drugs. Protection against spontaneous apoptosis could also be induced by coculturing the CLL B cells with normal dendritic cells (DCs) or with a CD40-ligand (CD154)-expressing fibroblast cell line. Examination of the expression of several apoptosis-regulatory proteins revealed that coculture with HK cells or DCs induced up-regulation of the antiapoptotic Bcl-2 family protein Mcl-1 in CLL B cells, whereas CD40 ligation increased expression of Bcl-X(L). Cell-cell contact was required for HK-induced protection, and introducing neutralizing antibodies against various adhesion molecules showed that CD44 was involved in HK-mediated survival, whereas CD40, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) were not. Anti-CD44 antibodies also blocked Mcl-1 induction by HK cells. Mcl-1 antisense oligonucleotides reduced leukemia cell expression of Mcl-1, and significantly suppressed HK-induced protection against apoptosis, whereas control oligonucleotides had no effect. Thus, HK cells protect CLL B cells against apoptosis, at least in part through a CD44-dependent mechanism involving up-regulation of Mcl-1, and this mechanism is distinct from that achieved by CD40 ligation. Consequently, the particular antiapoptotic proteins important for CLL survival may vary depending on the microenvironment. | 12176902
 |