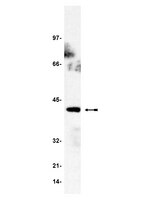
Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor-independent TCR signaling hub.
Roncagalli, R; Hauri, S; Fiore, F; Liang, Y; Chen, Z; Sansoni, A; Kanduri, K; Joly, R; Malzac, A; Lähdesmäki, H; Lahesmaa, R; Yamasaki, S; Saito, T; Malissen, M; Aebersold, R; Gstaiger, M; Malissen, B
Nature immunology
15
384-92
2014
요약 표시
T cell antigen receptor (TCR)-mediated activation of T cells requires the interaction of dozens of proteins. Here we used quantitative mass spectrometry and activated primary CD4(+) T cells from mice in which a tag for affinity purification was knocked into several genes to determine the composition and dynamics of multiprotein complexes that formed around the kinase Zap70 and the adaptors Lat and SLP-76. Most of the 112 high-confidence time-resolved protein interactions we observed were previously unknown. The surface receptor CD6 was able to initiate its own signaling pathway by recruiting SLP-76 and the guanine nucleotide-exchange factor Vav1 regardless of the presence of Lat. Our findings provide a more complete model of TCR signaling in which CD6 constitutes a signaling hub that contributes to the diversification of TCR signaling. | 24584089
 |
Luminal leptin inhibits L-glutamine transport in rat small intestine: involvement of ASCT2 and B0AT1.
Ducroc R, Sakar Y, Fanjul C, Barber A, Bado A, Lostao MP
Am J Physiol Gastrointest Liver Physiol
299
G179-85. Epub 2010 May 6.
2010
요약 표시
l-glutamine is the primary metabolic fuel for enterocytes. Glutamine from the diet is transported into the absorptive cells by two sodium-dependent neutral amino acid transporters present at the apical membrane: ASCT2/SLC1A5 and B(0)AT1/SLC6A19. We have demonstrated that leptin is secreted into the stomach lumen after a meal and modulates the transport of sugars after binding to its receptors located at the brush border of the enterocytes. The present study was designed to address the effect of luminal leptin on Na(+)-dependent glutamine (Gln) transport in rat intestine and identify the transporters involved. We found that 0.2 nM leptin inhibited uptake of Gln and phenylalanine (Phe) (substrate of B(0)AT1) using everted intestinal rings. In Ussing chambers, 10 mM Gln absorption followed as Na(+)-induced short-circuit current was inhibited by leptin in a dose-dependent manner (maximum inhibition at 10 nM; I(C50) = approximately 0.1 nM). Phe absorption was also decreased by leptin. Western blot analysis after 3-min incubation of the intestinal loops with 10 mM Gln, showed marked increase of ASCT2 and B(0)AT1 protein in the brush-border membrane that was reduced by rapid preincubation of the intestinal lumen with 1 nM leptin. Similarly, the increase in ASCT2 and B(0)AT1 gene expression induced by 60-min incubation of the intestine with 10 mM Gln was strongly reduced after a short preincubation period with leptin. Altogether these data demonstrate that, in rat, leptin controls the active Gln entry through reduction of both B(0)AT1 and ASCT2 proteins traffic to the apical plasma membrane and modulation of their gene expression. | 20448142
 |
In vivo significance of ITK-SLP-76 interaction in cytokine production.
Juris A Grasis,David M Guimond,Nicholas R Cam,Krystal Herman,Paola Magotti,John D Lambris,Constantine D Tsoukas
Molecular and cellular biology
30
2010
요약 표시
In vitro data have suggested that activation of the inducible T-cell kinase (ITK) requires an interaction with the adaptor protein SLP-76. One means for this interaction involves binding of the ITK SH3 domain to the polyproline-rich (PR) region of SLP-76. However, the biological significance of this association in live cells and the consequences of its disruption have not been demonstrated. Here, we utilized a polyarginine-rich, cell-permeable peptide that represents the portion of the SLP-76 PR region that interacts with the ITK SH3 domain as a competitive inhibitor to disrupt the association between ITK and SLP-76 in live cells. We demonstrate that treatment of cells with this peptide, by either in vitro incubation or intraperitoneal injection of the peptide in mice, inhibits the T-cell receptor (TCR)-induced association between ITK and SLP-76, recruitment and transphosphorylation of ITK, actin polarization at the T-cell contact site, and expression of Th2 cytokines. The inhibition is specific, as indicated by lack of effects by the polyarginine vehicle alone or a scrambled sequence of the cargo peptide. In view of the role of ITK as a regulator of Th2 cytokine expression, the data underscore the significance of ITK as a target for pharmacological intervention. 기사 전문 | 20457812
 |
Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell-mediated immune responses.
Jr-Wen Shui, Jonathan S Boomer, Jin Han, Jun Xu, Gregory A Dement, Guisheng Zhou, Tse-Hua Tan
Nature immunology
8
84-91
2007
요약 표시
HPK1 is a Ste20-related serine-threonine kinase that inducibly associates with the adaptors SLP-76 and Gads after T cell receptor (TCR) signaling. Here, HPK1 deficiency resulted in enhanced TCR-induced phosphorylation of SLP-76, phospholipase C-gamma1 and the kinase Erk, more-persistent calcium flux, and increased production of cytokines and antigen-specific antibodies. Furthermore, HPK1-deficient mice were more susceptible to experimental autoimmune encephalomyelitis. Although the interaction between SLP-76 and Gads was unaffected, the inducible association of SLP-76 with 14-3-3tau (a phosphorylated serine-binding protein and negative regulator of TCR signaling) was reduced in HPK1-deficient T cells after TCR stimulation. HPK1 phosphorylated SLP-76 and induced the interaction of SLP-76 with 14-3-3tau. Our results indicate that HPK1 negatively regulates TCR signaling and T cell-mediated immune responses. | 17115060
 |