SAFETY AND TOLERABILITY OF MRI-GUIDED INFUSION OF AAV2-hAADC INTO THE MID-BRAIN OF NON-HUMAN PRIMATE.
San Sebastian, W; Kells, AP; Bringas, J; Samaranch, L; Hadaczek, P; Ciesielska, A; Macayan, M; Pivirotto, PJ; Forsayeth, J; Osborne, S; Wright, JF; Green, F; Heller, G; Bankiewicz, KS
Molecular therapy. Methods & clinical development
3
2014
요약 표시
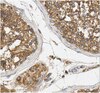
Aromatic L-amino acid decarboxylase (AADC) deficiency is a rare, autosomal-recessive neurological disorder caused by mutations in the DDC gene that leads to an inability to synthesize catecholamines and serotonin. As a result, patients suffer compromised development, particularly in motor function. A recent gene replacement clinical trial explored putaminal delivery of recombinant adeno-associated virus serotype 2 vector encoding human AADC (AAV2-hAADC) in AADC-deficient children. Unfortunately, patients presented only modest amelioration of motor symptoms, which authors acknowledged could be due to insufficient transduction of putamen. We hypothesize that, with the development of a highly accurate MRI-guided cannula placement technology, a more effective approach might be to target the affected mid-brain neurons directly. Transduction of AADC-deficient dopaminergic neurons in the substantia nigra and ventral tegmental area with locally infused AAV2-hAADC would be expected to lead to restoration of normal dopamine levels in affected children. The objective of this study was to assess the long-term safety and tolerability of bilateral AAV2-hAADC MRI-guided pressurized infusion into the mid-brain of non-human primates. Animals received either vehicle, low or high AAV2-hAADC vector dose and were euthanized 1, 3 or 9 months after surgery. Our data indicate that effective mid-brain transduction was achieved without untoward effects. | Immunohistochemistry | 25541617
 |
Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses.
Ciesielska, A; Hadaczek, P; Mittermeyer, G; Zhou, S; Wright, JF; Bankiewicz, KS; Forsayeth, J
Molecular therapy : the journal of the American Society of Gene Therapy
21
158-66
2013
요약 표시
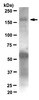
There is considerable interest in the use of adeno-associated virus serotype 9 (AAV9) for neurological gene therapy partly because of its ability to cross the blood-brain barrier to transduce astrocytes and neurons. This raises the possibility that AAV9 might also transduce antigen-presenting cells (APC) in the brain and provoke an adaptive immune response. We tested this hypothesis by infusing AAV9 vectors encoding foreign antigens, namely human aromatic L-amino acid decarboxylase (hAADC) and green fluorescent protein (GFP), into rat brain parenchyma. Over ensuing weeks, both vectors elicited a prominent inflammation in transduced brain regions associated with upregulation of MHC II in glia and associated lymphocytic infiltration. Transduction of either thalamus or striatum with AAV9-hAADC evinced a significant loss of neurons and induction of anti-hAADC antibodies. We conclude that AAV9 transduces APC in the brain and, depending on the immunogenicity of the transgene, can provoke a full immune response that mediates significant brain pathology. We emphasize, however, that these observations do not preclude the use of AAV serotypes that can transduce APC. However, it does potentially complicate preclinical toxicology studies in which non-self proteins are expressed at a level sufficient to trigger cell-mediated and humoral immune responses. | | 22929660
 |
Intrarenal dopamine inhibits progression of diabetic nephropathy.
Zhang, MZ; Yao, B; Yang, S; Yang, H; Wang, S; Fan, X; Yin, H; Fogo, AB; Moeckel, GW; Harris, RC
Diabetes
61
2575-84
2012
요약 표시
The kidney has a local intrarenal dopaminergic system, and in the kidney, dopamine modulates renal hemodynamics, inhibits salt and fluid reabsorption, antagonizes the renin-angiotensin system, and inhibits oxidative stress. The current study examined the effects of alterations in the intrarenal dopaminergic system on kidney structure and function in models of type 1 diabetes. We studied catechol-O-methyl-transferase (COMT)(-/-) mice, which have increased renal dopamine production due to decreased dopamine metabolism, and renal transplantation was used to determine whether the effects seen with COMT deficiency were kidney-specific. To determine the effects of selective inhibition of intrarenal dopamine production, we used mice with proximal tubule deletion of aromatic amino acid decarboxylase (ptAADC(-/-)). Compared with wild-type diabetic mice, COMT(-/-) mice had decreased hyperfiltration, decreased macula densa cyclooxygenase-2 expression, decreased albuminuria, decreased glomerulopathy, and inhibition of expression of markers of inflammation, oxidative stress, and fibrosis. These differences were also seen in diabetic mice with a transplanted kidney from COMT(-/-) mice. In contrast, diabetic ptAADC(-/-) mice had increased nephropathy. Our study demonstrates an important role of the intrarenal dopaminergic system to modulate the development and progression of diabetic kidney injury and indicate that the decreased renal dopamine production may have important consequences in the underlying pathogenesis of diabetic nephropathy. | | 22688335
 |
Safety and tolerability of magnetic resonance imaging-guided convection-enhanced delivery of AAV2-hAADC with a novel delivery platform in nonhuman primate striatum.
San Sebastian, W; Richardson, RM; Kells, AP; Lamarre, C; Bringas, J; Pivirotto, P; Salegio, EA; Dearmond, SJ; Forsayeth, J; Bankiewicz, KS
Human gene therapy
23
210-7
2012
요약 표시
Degeneration of nigrostriatal neurons in Parkinson's disease (PD) causes progressive loss of aromatic l-amino acid decarboxylase (AADC), the enzyme that converts levodopa (l-DOPA) into dopamine in the striatum. Because loss of this enzyme appears to be a major driver of progressive impairment of response to the mainstay drug, l-DOPA, one promising approach has been to use gene therapy to restore AADC activity in the human putamen and thereby restore normal l-DOPA response in patients with PD. An open-label phase I clinical trial of this approach in patients with PD provided encouraging signs of improvement in Unified Parkinson's Disease Rating Scale scores and reductions in antiparkinsonian medications. However, such improvement was modest compared with the results previously reported in parkinsonian rhesus macaques. The reason for this discrepancy may have been that the relatively small volume of vector infused in the clinical study restricted the distribution of AADC expression, such that only about 20% of the postcommissural putamen was covered, as revealed by l-[3-(18)F]-α-methyltyrosine-positron emission tomography. To achieve more quantitative distribution of vector, we have developed a visual guidance system for parenchymal infusion of AAV2. The purpose of the present study was to evaluate the combined magnetic resonance imaging-guided delivery system with AAV2-hAADC under conditions that approximate the intended clinical protocol. Our data indicate that this approach directed accurate cannula placement and effective vector distribution without inducing any untoward effects in nonhuman primates infused with a high dose of AAV2-hAADC. | | 22017504
 |
Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury.
Yang, S; Yao, B; Zhou, Y; Yin, H; Zhang, MZ; Harris, RC
American journal of physiology. Renal physiology
302
F742-9
2012
요약 표시
It is well-recognized that excessive angiotensin II (ANG II) can mediate progressive renal injury. Previous studies by us and others have indicated that dopamine may modulate actions of ANG II in the kidney. The current studies investigated whether altering intrarenal dopamine levels affected ANG II-mediated renal fibrosis. We utilized a model of increased intrarenal dopamine, catechol-O-methyl-transferase knockout (COMT KO) mice, which have increased kidney dopamine levels due to deletion of a major intrarenal dopamine-metabolizing enzyme. In wild-type mice, chronic ANG II infusion increased renal expression of both of the major dopamine-metabolizing enzymes, COMT and monoamine oxidase. After 8 wk of ANG II infusion, there were no significant differences in blood pressure between wild-type and COMT KO mice. Compared with wild-type, COMT KO mice had decreased albuminuria and tubulointerstitial injury. In response to ANG II infusion, there was decreased expression of both glomerular and tubulointerstitial injury markers (fibronectin, connective tissue growth factor, fibroblast-specific protein-1, collagen I, podocyte vascular endothelial growth factor) in COMT KO mice. We recently reported that ANG II-mediated tubulointerstitial fibrosis is mediated by src-dependent epidermal growth factor receptor (EGFR) activation. In aromatic l-amino acid decarboxylase knockout (AADC KO) mice, a model of intrarenal dopamine deficiency due to selective proximal tubule AADC deletion, which inhibits intrarenal dopamine synthesis, ANG II infusion further increased expression of p-src and pTyr845-EGFR. In contrast, their expression was markedly attenuated in COMT KO mice. These results demonstrate a role for intrarenal dopamine to buffer the detrimental effects of ANG II upon the kidney. | | 22169008
 |
Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice.
Zhang, MZ; Yao, B; Wang, S; Fan, X; Wu, G; Yang, H; Yin, H; Yang, S; Harris, RC
The Journal of clinical investigation
121
2845-54
2011
요약 표시
In addition to its role as an essential neurotransmitter, dopamine serves important physiologic functions in organs such as the kidney. Although the kidney synthesizes dopamine through the actions of aromatic amino acid decarboxylase (AADC) in the proximal tubule, previous studies have not discriminated between the roles of extrarenal and intrarenal dopamine in the overall regulation of renal function. To address this issue, we generated mice with selective deletion of AADC in the kidney proximal tubules (referred to herein as ptAadc-/- mice), which led to selective decreases in kidney and urinary dopamine. The ptAadc-/- mice exhibited increased expression of nephron sodium transporters, decreased natriuresis and diuresis in response to l-dihydroxyphenylalanine, and decreased medullary COX-2 expression and urinary prostaglandin E2 excretion and developed salt-sensitive hypertension. They had increased renin expression and altered renal Ang II receptor (AT) expression, with increased AT1b and decreased AT2 and Mas expression, associated with increased renal injury in response to Ang II. They also exhibited a substantially shorter life span compared with that of wild-type mice. These results demonstrate the importance of the intrarenal dopaminergic system in salt and water homeostasis and blood pressure control. Decreasing intrarenal dopamine subjects the kidney to unbuffered responses to Ang II and results in the development of hypertension and a dramatic decrease in longevity. | | 21701066
 |
Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation.
Wright, JF; Le, T; Prado, J; Bahr-Davidson, J; Smith, PH; Zhen, Z; Sommer, JM; Pierce, GF; Qu, G
Molecular therapy : the journal of the American Society of Gene Therapy
12
171-8
2005
요약 표시
Aggregation of recombinant AAV2 results in reduced yield during purification and may have deleterious effects on vector transduction efficiency, biodistribution and immunogenicity following in vivo administration. Studies to elucidate the mechanism of vector aggregation and methods to prevent its occurrence are reported. In excipient screening studies, the sugars sorbitol, sucrose, mannitol, trehalose, or glycerol at concentrations of up to 5% (w/v), or surfactants Tween 80 or Pluronic F68, did not prevent aggregation. Aggregation was prevented by the use of various salts at concentrations corresponding to solution ionic strengths of greater than 200 mM. AAV2 vectors purified by double cesium chloride gradient centrifugation, cation-exchange chromatography, or combined chromatography and gradient centrifugation each demonstrated a similar requirement for ionic strength to prevent aggregation. AAV2 vectors concentrated to 6.7 x 10(13) vector genome (vg)/mL in neutral-buffered isotonic saline resulted in 59+/-6.0% recovery of nonaggregated material compared to 96+/-4.4% recovery in an isotonic formulation with elevated ionic strength. The latter showed no aggregation following storage or after 10 freeze-thaw cycles at -20 degrees C. AAV2 vectors stored for an extended period in an elevated ionic strength formulation retained a high infectivity titer (13 vg/infectious unit) and transduction efficiency. Nuclease digestion of purified AAV2 vectors reduced aggregation, implicating trace amounts of vector surface nucleic acids in interparticle binding. | | 15963933
 |