Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent.
McKnight, A; Wilkinson, D; Simmons, G; Talbot, S; Picard, L; Ahuja, M; Marsh, M; Hoxie, JA; Clapham, PR
Journal of virology
71
1692-6
1997
요약 표시
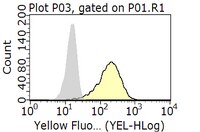
CXCR4 (also termed fusin, LESTR, or HUMSTR) is a member of the G-protein-coupled chemokine receptor family with seven membrane-spanning domains. CXCR4 acts as a coreceptor for syncytium-inducing human immunodeficiency virus type 1 (HIV-1) strains, conferring entry into CD4+ cells. We show here that a novel mouse monoclonal antibody (12G5) that recognizes CXCR4 blocked cell-to-cell fusion and cell free-virus infection of CXCR4+ CD4+ RD rhabdomyosarcoma cells by seven HIV-1 and HIV-2 strains that had various cell tropisms for different CD4+ human cell types. Yet the majority of the members of the same virus panel resisted 12G5 inhibition on T-cell lines. When inhibition was observed on these cell types, it was both cell type and virus strain dependent. In at least one situation, 12G5 failed to block LAI infection of cells expressing CXCR4 as the only available coreceptor. Our observations suggest that CXCR4 could be processed or presented differently depending on the cell type, allowing some strains to evade 12G5 inhibition. Alternatively, since several of the viruses could infect certain CXCR4- CD4+ cell lines, it is conceivable that alternative coreceptors are active, enabling individual HIV strains to choose between compatible coreceptors during entry into cells. Moreover, the strain dependency of 12G5 inhibition implies that the interaction of different HIVs with CXCR4 varies. | 8995702
 |
The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes.
Bleul, CC; Wu, L; Hoxie, JA; Springer, TA; Mackay, CR
Proceedings of the National Academy of Sciences of the United States of America
94
1925-30
1997
요약 표시
The chemokine receptors CXCR4 and CCR5 function as coreceptors for HIV-1 entry into CD4+ cells. During the early stages of HIV infection, viral isolates tend to use CCR5 for viral entry, while later isolates tend to use CXCR4. The pattern of expression of these chemokine receptors on T cell subsets and their regulation has important implications for AIDS pathogenesis and lymphocyte recirculation. A mAb to CXCR4, 12G5, showed partial inhibition of chemotaxis and calcium influx induced by SDF-1, the natural ligand of CXCR4. 12G5 stained predominantly the naive, unactivated CD26(low) CD45RA+ CD45R0- T lymphocyte subset of peripheral blood lymphocytes. In contrast, a mAb specific for CCR5, 5C7, stained CD26(high) CD45RA(low) CD45R0+ T lymphocytes, a subset thought to represent previously activated/memory cells. CXCR4 expression was rapidly up-regulated on peripheral blood mononuclear cells during phytohemagglutinin stimulation and interleukin 2 priming, and responsiveness to SDF-1 increased simultaneously. CCR5 expression, however, showed only a gradual increase over 12 days of culture with interleukin 2, while T cell activation with phytohemagglutinin was ineffective. Taken together, the data suggest distinct functions for the two receptors and their ligands in the migration of lymphocyte subsets through lymphoid and nonlymphoid tissues. Furthermore, the largely reciprocal expression of CXCR4 and CCR5 among peripheral blood T cells implies distinct susceptibility of T cell subsets to viral entry by T cell line-tropic versus macrophage-tropic strains during the course of HIV infection. | 9050881
 |
CD4-independent infection by HIV-2 is mediated by fusin/CXCR4.
Endres, MJ; Clapham, PR; Marsh, M; Ahuja, M; Turner, JD; McKnight, A; Thomas, JF; Stoebenau-Haggarty, B; Choe, S; Vance, PJ; Wells, TN; Power, CA; Sutterwala, SS; Doms, RW; Landau, NR; Hoxie, JA
Cell
87
745-56
1996
요약 표시
Several members of the chemokine receptor family have been shown to function in association with CD4 to permit HIV-1 entry and infection. However, the mechanism by which these molecules serve as CD4-associated cofactors is unclear. In the present report, we show that one member of this family, termed Fusin/ CXCR4, is able to function as an alternative receptor for some isolates of HIV-2 in the absence of CD4. This conclusion is supported by the finding that (1) CD4-independent infection by these viruses is inhibited by an anti-Fusin monoclonal antibody, (2) Fusin expression renders human and nonhuman CD4-negative cell lines sensitive to HIV-2-induced syncytium induction and/or infection, and (3) Fusin is selectively down-regulated from the cell surface following HIV-2 infection. The finding that one chemokine receptor can function as a primary viral receptor strongly suggests that the HIV envelope glycoprotein contains a binding site for these proteins and that differences in the affinity and/or the availability of this site can extend the host range of these viruses to include a number of CD4-negative cell types. | 8929542
 |