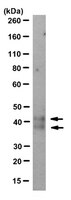
CD1d degradation in Chlamydia trachomatis-infected epithelial cells is the result of both cellular and chlamydial proteasomal activity.
Kawana, K; Quayle, AJ; Ficarra, M; Ibana, JA; Shen, L; Kawana, Y; Yang, H; Marrero, L; Yavagal, S; Greene, SJ; Zhang, YX; Pyles, RB; Blumberg, RS; Schust, DJ
The Journal of biological chemistry
282
7368-75
2007
요약 표시
Chlamydia trachomatis is an obligate intracellular pathogen that can persist in the urogenital tract. Mechanisms by which C. trachomatis evades clearance by host innate immune responses are poorly described. CD1d is MHC-like, is expressed by epithelial cells, and can signal innate immune responses by NK and NKT cells. Here we demonstrate that C. trachomatis infection down-regulates surface-expressed CD1d in human penile urethral epithelial cells through proteasomal degradation. A chlamydial proteasome-like activity factor (CPAF) interacts with the CD1d heavy chain, and CPAF-associated CD1d heavy chain is then ubiquitinated and directed along two distinct proteolytic pathways. The degradation of immature glycosylated CD1d was blocked by the proteasome inhibitor lactacystin but not by MG132, indicating that degradation was not via the conventional proteasome. In contrast, the degradation of non-glycosylated CD1d was blocked by lactacystin and MG132, consistent with conventional cellular cytosolic degradation of N-linked glycoproteins. Immunofluorescent microscopy confirmed the interruption of CD1d trafficking to the cell surface, and the dislocation of CD1d heavy chains into both the cellular cytosol and the chlamydial inclusion along with cytosolic CPAF. C. trachomatis targeted CD1d toward two distinct proteolytic pathways. Decreased CD1d surface expression may help C. trachomatis evade detection by innate immune cells and may promote C. trachomatis persistence. | 17215251
 |
Biochemical characterization of CD1d expression in the absence of beta2-microglobulin.
Kim, HS; Garcia, J; Exley, M; Johnson, KW; Balk, SP; Blumberg, RS
The Journal of biological chemistry
274
9289-95
1999
요약 표시
CD1d is a major histocompatibility complex class I-like molecule that exhibits a distinct antigen processing pathway that functions in the presentation of hydrophobic antigens to T cells. CD1d has been previously shown to be expressed on the cell surface of human intestinal epithelial cell lines in vivo and a transfected cell line in vitro independently of beta2-microglobulin (beta2m). To define the relationship between CD1d and beta2m and characterize the biochemical structure of CD1d in the absence of beta2m, we have used a newly generated series of CD1d transfectants and CD1d-specific antibodies. These studies show that in the absence of beta2m, CD1d is expressed on the cell surface as a 45-kDa glycoprotein that is sensitive to endoglycosidase-H and is reduced to 37-kDa after N-glycanase digestion. In contrast, in the presence of beta2m, CD1d is expressed on the cell surface as a 48-kDa endoglycosidase-H-resistant glycoprotein. Pulse-chase metabolic labeling studies demonstrate that acquisition of endoglycosidase-H resistance of CD1d is observed in the presence of beta2m but not in the absence of beta2m even after a 24-h chase period. Thus, CD1d is able to be transported to the cell surface independently of beta2m; however, in the absence of beta2m, the glycosylation pattern of CD1d is altered and consistent with an immature glycoprotein. | 10092605
 |