Spatial and temporally restricted expression of chemokines and chemokine receptors in the developing human kidney.
Hermann-Josef Gröne, Clemens D Cohen, Elisabeth Gröne, Claudia Schmidt, Matthias Kretzler, Detlef Schlöndorff, Peter J Nelson
Journal of the American Society of Nephrology : JASN
13
957-67
2002
요약 표시
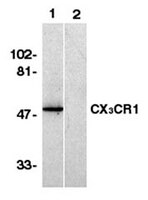
The directed migration of cells, cell-cell adhesion, and the control of proliferation are key events during metanephric development. The chemokines are a family of proteins that selectively control aspects of cell migration, activation, proliferation, and adhesion. The expression of a series of chemokines and chemokine receptors during human renal development was investigated by using immunohistochemical analyses and real-time reverse transcription-PCR assays of defined laser-microdissected metanephric structures. The results demonstrate that mononuclear cell-like cells within the nephrogenic blastema focally express interferon-inducible protein-10/CXCL10, a ligand for CXCR3. Mononuclear-like cells dispersed through the developing organ express CX(3)CR1. Expression of CXCR4, the receptor for stromal cell-derived factor-1/CXCL12, is also limited to stromal CD34-positive cells. In contrast, the expression of stromal cell-derived factor-1/CXCL12, fractalkine, and CXCR3 is first observed in the comma- or S-shaped body stage. The intensity of this expression becomes stronger in the capillary loop stage, and expression is mainly observed in the mesangial stalk and endothelial cells of the glomeruli. These proteins may play modulatory roles in kidney development. Because genes that are expressed during ontogeny often play a role in tissue regeneration, these embryonal chemokine/chemokine receptor patterns may be important in renal injury and repair. | 11912255
 |
Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion.
Imai, T, et al.
Cell, 91: 521-30 (1997)
1997
요약 표시
Leukocyte trafficking at the endothelium requires both cellular adhesion molecules and chemotactic factors. Fractalkine, a novel transmembrane molecule with a CX3C-motif chemokine domain atop a mucin stalk, induces both adhesion and migration of leukocytes. Here we identify a seven-transmembrane high-affinity receptor for fractalkine and show that it mediates both the adhesive and migratory functions of fractalkine. The receptor, now termed CX3CR1, requires pertussis toxin-sensitive G protein signaling to induce migration but not to support adhesion, which also occurs without other adhesion molecules but requires the architecture of a chemokine domain atop the mucin stalk. Natural killer cells predominantly express CX3CR1 and respond to fractalkine in both migration and adhesion. Thus, fractalkine and CX3CR1 represent new types of leukocyte trafficking regulators, performing both adhesive and chemotactic functions. | 9390561
 |
The orphan G-protein-coupled receptor-encoding gene V28 is closely related to genes for chemokine receptors and is expressed in lymphoid and neural tissues.
Raport, C J, et al.
Gene, 163: 295-9 (1995)
1995
요약 표시
A polymerase chain reaction (PCR) strategy with degenerate primers was used to identify novel G-protein-coupled receptor-encoding genes from human genomic DNA. One of the isolated clones, termed V28, showed high sequence similarity to the genes encoding human chemokine receptors for monocyte chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1 alpha (MIP-1 alpha)/RANTES, and to the rat orphan receptor-encoding gene RBS11. When RNA was analyzed by Northern blot, V28 was found to be most highly expressed in neural and lymphoid tissues. Myeloid cell lines, particularly THP.1 cells, showed especially high expression of V28. We have mapped V28 to human chromosome 3p21-3pter, near the MIP-1 alpha/RANTES receptor-encoding gene. | 7590284
 |
Cloning, chromosomal localization, and RNA expression of a human beta chemokine receptor-like gene.
Combadiere, C, et al.
DNA Cell Biol., 14: 673-80 (1995)
1995
요약 표시
A human cDNA encoding a putative G protein-coupled receptor designated chemokine beta receptor-like 1 (CMKBRL1) was isolated from an eosinophilic leukemia library. Its deduced sequence is approximately 40% identical to previously cloned receptors for the beta chemokines macrophage inflammatory protein-1 alpha (MIP-1 alpha), RANTES, and monocyte chemoattractant protein-1 (MCP-1), which are chemoattractants for blood leukocytes, and is 83% identical to the product of the orphan rat cDNA RBS 11. Like the MIP-1 alpha/RANTES receptor, CMK-BRL1 is encoded by a small, single-copy gene that maps to chromosome 3p21 and is expressed in leukocytes. However, two screening assays with a broad panel of chemokines failed to identify its ligand. CMKBRL1 mRNA was detectable by Northern blot hybridization in neutrophils and monocytes, but not eosinophils, and was also found in eight solid organs that were tested with particularly high expression in brain. The RNA distribution of the known beta chemokine receptors was overlapping but distinct from that of CMKBRL1. MIP-1 alpha/RANTES receptor mRNA was detectable in neutrophils, monocytes, eosinophils, and in all eight solid organs tested, with particularly high expression in placenta, lung, and liver. MCP-1 receptor mRNA was found in monocytes, lung, liver, and pancreas. These results suggest that the ligand for the putative CMKBRL1 receptor is a beta chemokine that targets both neutrophils and monocytes. Moreover, the RNA distributions suggest that CMKBRL1, the MIP-1 alpha/RANTES receptor, and the MCP-1 receptor may have both overlapping and distinct biological roles. | 7646814
 |
cDNA cloning of a G-protein-coupled receptor expressed in rat spinal cord and brain related to chemokine receptors.
Harrison, J K, et al.
Neurosci. Lett., 169: 85-9 (1994)
1994
요약 표시
A series of cDNAs and a genomic clone (named RBS11) were isolated, from a variety of rat brainstem, pituitary and/or spinal cord cDNA libraries and a genomic library by low-stringency hybridization screening with a rat angiotensin receptor cDNA. The RBS11 protein, as conceptualized from these DNAs, is a novel member of the rhodopsin family of the G-protein-coupled receptor (GCR) superfamily. Comparison of RBS11 to other members of the GCR superfamily suggests that the RBS11 protein might be a receptor for a peptide ligand in the chemokine family. The RBS11 protein sequence is unusual in that it is without N-linked glycosylation consensus sequences in the putative exofacial regions. Northern analysis indicates that the mRNA for RBS11 accumulates widely and unevenly in the adult rat, with the mRNA being most prominent in extracts of spinal cord, brain, kidney, gut, uterus and testes. | 8047298
 |