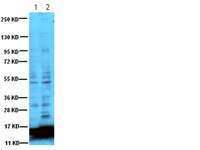
The HDAC6/APOBEC3G complex regulates HIV-1 infectiveness by inducing Vif autophagic degradation.
Valera, MS; de Armas-Rillo, L; Barroso-González, J; Ziglio, S; Batisse, J; Dubois, N; Marrero-Hernández, S; Borel, S; García-Expósito, L; Biard-Piechaczyk, M; Paillart, JC; Valenzuela-Fernández, A
Retrovirology
12
53
2015
요약 표시
Human immunodeficiency virus type 1 (HIV-1) has evolved a complex strategy to overcome the immune barriers it encounters throughout an organism thanks to its viral infectivity factor (Vif), a key protein for HIV-1 infectivity and in vivo pathogenesis. Vif interacts with and promotes "apolipoprotein B mRNA-editing enzyme-catalytic, polypeptide-like 3G" (A3G) ubiquitination and subsequent degradation by the proteasome, thus eluding A3G restriction activity against HIV-1.We found that cellular histone deacetylase 6 (HDAC6) directly interacts with A3G through its C-terminal BUZ domain (residues 841-1,215) to undergo a cellular co-distribution along microtubules and cytoplasm. The HDAC6/A3G complex occurs in the absence or presence of Vif, competes for Vif-mediated A3G degradation, and accounts for A3G steady-state expression level. In fact, HDAC6 directly interacts with and promotes Vif autophagic clearance, thanks to its C-terminal BUZ domain, a process requiring the deacetylase activity of HDAC6. HDAC6 degrades Vif without affecting the core binding factor β (CBF-β), a Vif-associated partner reported to be key for Vif- mediated A3G degradation. Thus HDAC6 antagonizes the proviral activity of Vif/CBF-β-associated complex by targeting Vif and stabilizing A3G. Finally, in cells producing virions, we observed a clear-cut correlation between the ability of HDAC6 to degrade Vif and to restore A3G expression, suggesting that HDAC6 controls the amount of Vif incorporated into nascent virions and the ability of HIV-1 particles of being infectious. This effect seems independent on the presence of A3G inside virions and on viral tropism.Our study identifies for the first time a new cellular complex, HDAC6/A3G, involved in the autophagic degradation of Vif, and suggests that HDAC6 represents a new antiviral factor capable of controlling HIV-1 infectiveness by counteracting Vif and its functions. | | | 26105074
 |
Rational design and validation of a Tip60 histone acetyltransferase inhibitor.
Gao, C; Bourke, E; Scobie, M; Famme, MA; Koolmeister, T; Helleday, T; Eriksson, LA; Lowndes, NF; Brown, JA
Scientific reports
4
5372
2014
요약 표시
Histone acetylation is required for many aspects of gene regulation, genome maintenance and metabolism and dysfunctional acetylation is implicated in numerous diseases, including cancer. Acetylation is regulated by histone acetyltransferases (HATs) and histone deacetylases and currently, few general HAT inhibitors have been described. We identified the HAT Tip60 as an excellent candidate for targeted drug development, as Tip60 is a key mediator of the DNA damage response and transcriptional co-activator. Our modeling of Tip60 indicated that the active binding pocket possesses opposite charges at each end, with the positive charges attributed to two specific side chains. We used structure based drug design to develop a novel Tip60 inhibitor, TH1834, to fit this specific pocket. We demonstrate that TH1834 significantly inhibits Tip60 activity in vitro and treating cells with TH1834 results in apoptosis and increased unrepaired DNA damage (following ionizing radiation treatment) in breast cancer but not control cell lines. Furthermore, TH1834 did not affect the activity of related HAT MOF, as indicated by H4K16Ac, demonstrating specificity. The modeling and validation of the small molecule inhibitor TH1834 represents a first step towards developing additional specific, targeted inhibitors of Tip60 that may lead to further improvements in the treatment of breast cancer. | Western Blotting | Chicken | 24947938
 |
Tubulin acetyltransferase αTAT1 destabilizes microtubules independently of its acetylation activity.
Kalebic, N; Martinez, C; Perlas, E; Hublitz, P; Bilbao-Cortes, D; Fiedorczuk, K; Andolfo, A; Heppenstall, PA
Molecular and cellular biology
33
1114-23
2013
요약 표시
Acetylation of α-tubulin at lysine 40 (K40) is a well-conserved posttranslational modification that marks long-lived microtubules but has poorly understood functional significance. Recently, αTAT1, a member of the Gcn5-related N-acetyltransferase superfamily, has been identified as an α-tubulin acetyltransferase in ciliated organisms. Here, we explored the function of αTAT1 with the aim of understanding the consequences of αTAT1-mediated microtubule acetylation. We demonstrate that α-tubulin is the major target of αTAT1 but that αTAT1 also acetylates itself in a regulatory mechanism that is required for effective modification of tubulin. We further show that in mammalian cells, αTAT1 promotes microtubule destabilization and accelerates microtubule dynamics. Intriguingly, this effect persists in an αTAT1 mutant with no acetyltransferase activity, suggesting that interaction of αTAT1 with microtubules, rather than acetylation per se, is the critical factor regulating microtubule stability. Our data demonstrate that αTAT1 has cellular functions that extend beyond its classical enzymatic activity as an α-tubulin acetyltransferase. | | | 23275437
 |
HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates.
Rajendran, P; Kidane, AI; Yu, TW; Dashwood, WM; Bisson, WH; Löhr, CV; Ho, E; Williams, DE; Dashwood, RH
Epigenetics
8
612-23
2013
요약 표시
Histone deacetylases (HDACs) and acetyltransferases have important roles in the regulation of protein acetylation, chromatin dynamics and the DNA damage response. Here, we show in human colon cancer cells that dietary isothiocyanates (ITCs) inhibit HDAC activity and increase HDAC protein turnover with the potency proportional to alkyl chain length, i.e., AITC less than sulforaphane (SFN) less than 6-SFN less than 9-SFN. Molecular docking studies provided insights into the interactions of ITC metabolites with HDAC3, implicating the allosteric site between HDAC3 and its co-repressor. ITCs induced DNA double-strand breaks and enhanced the phosphorylation of histone H2AX, ataxia telangiectasia and Rad3-related protein (ATR) and checkpoint kinase-2 (CHK2). Depending on the ITC and treatment conditions, phenotypic outcomes included cell growth arrest, autophagy and apoptosis. Coincident with the loss of HDAC3 and HDAC6, as well as SIRT6, ITCs enhanced the acetylation and subsequent degradation of critical repair proteins, such as CtIP, and this was recapitulated in HDAC knockdown experiments. Importantly, colon cancer cells were far more susceptible than non-cancer cells to ITC-induced DNA damage, which persisted in the former case but was scarcely detectable in non-cancer colonic epithelial cells under the same conditions. Future studies will address the mechanistic basis for dietary ITCs preferentially exploiting HDAC turnover mechanisms and faulty DNA repair pathways in colon cancer cells vs. normal cells. | | | 23770684
 |
PSG gene expression is up-regulated by lysine acetylation involving histone and nonhistone proteins.
Camolotto, SA; Racca, AC; Ridano, ME; Genti-Raimondi, S; Panzetta-Dutari, GM
PloS one
8
e55992
2013
요약 표시
Lysine acetylation is an important post-translational modification that plays a central role in eukaryotic transcriptional activation by modifying chromatin and transcription-related factors. Human pregnancy-specific glycoproteins (PSG) are the major secreted placental proteins expressed by the syncytiotrophoblast at the end of pregnancy and represent early markers of cytotrophoblast differentiation. Low PSG levels are associated with complicated pregnancies, thus highlighting the importance of studying the mechanisms that control their expression. Despite several transcription factors having been implicated as key regulators of PSG gene family expression; the role of protein acetylation has not been explored.Here, we explored the role of acetylation on PSG gene expression in the human placental-derived JEG-3 cell line. Pharmacological inhibition of histone deacetylases (HDACs) up-regulated PSG protein and mRNA expression levels, and augmented the amount of acetylated histone H3 associated with PSG 5'regulatory regions. Moreover, PSG5 promoter activation mediated by Sp1 and KLF6, via the core promoter element motif (CPE, -147/-140), was markedly enhanced in the presence of the HDAC inhibitor trichostatin A (TSA). This effect correlated with an increase in Sp1 acetylation and KLF6 nuclear localization as revealed by immunoprecipitation and subcellular fractionation assays. The co-activators PCAF, p300, and CBP enhanced Sp1-dependent PSG5 promoter activation through their histone acetylase (HAT) function. Instead, p300 and CBP acetyltransferase domain was dispensable for sustaining co-activation of PSG5 promoter by KLF6.Results are consistent with a regulatory role of lysine acetylation on PSG expression through a relaxed chromatin state and an increase in the transcriptional activity of Sp1 and KLF6 following an augmented Sp1 acetylation and KLF6 nuclear localization. | | | 23418492
 |
Protein kinase D-HDAC5 signaling regulates erythropoiesis and contributes to erythropoietin cross-talk with GATA1.
Delehanty, LL; Bullock, GC; Goldfarb, AN
Blood
120
4219-28
2012
요약 표시
In red cell development, the differentiation program directed by the transcriptional regulator GATA1 requires signaling by the cytokine erythropoietin, but the mechanistic basis for this signaling requirement has remained unknown. Here we show that erythropoietin regulates GATA1 through protein kinase D activation, promoting histone deacetylase 5 (HDAC5) dissociation from GATA1, and subsequent GATA1 acetylation. Mice deficient for HDAC5 show resistance to anemic challenge and altered marrow responsiveness to erythropoietin injections. In ex vivo studies, HDAC5(-/-) progenitors display enhanced entry into and passage through the erythroid lineage, as well as evidence of erythropoietin-independent differentiation. These results reveal a molecular pathway that contributes to cytokine regulation of hematopoietic differentiation and offer a potential mechanism for fine tuning of lineage-restricted transcription factors by lineage-specific cytokines. | | | 22983445
 |
Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line.
Waby, JS; Chirakkal, H; Yu, C; Griffiths, GJ; Benson, RS; Bingle, CD; Corfe, BM
Molecular cancer
9
275
2010
요약 표시
Butyrate, a known histone deacetylase inhibitor (HDACi) and product of fibre fermentation, is postulated to mediate the protective effect of dietary fibre against colon cancer. The transcription factor Sp1 is a target of acetylation and is known to be associated with class I HDACs, including HDAC1. Sp1 is a ubiquitous transcription factor and Sp1-regulated genes include those involved in cell cycle regulation, apoptosis and lipogenesis: all major pathways in cancer development. The only known acetylated residue of Sp1 is lysine703 which resides in the DNA binding domain. Here we show that acetylated Sp1 loses p21- and bak-promoter -binding function in vitro. Furthermore treatment with a panel of HDAC inhibitors showed clustering of activities for a subset of inhibitors, causing G2 cell cycle arrest, Sp1 acetylation, p21 and Bak over-expression, all with very similar EC50 concentrations. These HDACi activities were not distributed according to the molecular class of compound. In order to mimic loss of binding, an siRNA strategy was used to reduce Sp1 expression. This resulted in altered expression of multiple elements of the p53/p21 pathway. Taken together our data suggest a mechanistic model for the chemopreventive actions of butyrate in colon epithelial cells, and provide new insight into the differential activities some classes of HDAC inhibitors. | Western Blotting | | 20950428
 |
Lovastatin-induced cholesterol depletion affects both apical sorting and endocytosis of aquaporin-2 in renal cells.
Procino G, Barbieri C, Carmosino M, Rizzo F, Valenti G, Svelto M
American journal of physiology. Renal physiology
298
F266-78. Epub 2009 Nov 18.
2010
요약 표시
Vasopressin causes the redistribution of the water channel aquaporin-2 (AQP2) from cytoplasmic storage vesicles to the apical plasma membrane of collecting duct principal cells, leading to urine concentration. The molecular mechanisms regulating the selective apical sorting of AQP2 are only partially uncovered. In this work, we investigate whether AQP2 sorting/trafficking is regulated by its association with membrane rafts. In both MCD4 cells and rat kidney, AQP2 preferentially associated with Lubrol WX-insoluble membranes regardless of its presence in the storage compartment or at the apical membrane. Block-and-release experiments indicate that 1) AQP2 associates with detergent-resistant membranes early in the biosynthetic pathway; 2) strong cholesterol depletion delays the exit of AQP2 from the trans-Golgi network. Interestingly, mild cholesterol depletion promoted a dramatic accumulation of AQP2 at the apical plasma membrane in MCD4 cells in the absence of forskolin stimulation. An internalization assay showed that AQP2 endocytosis was clearly reduced under this experimental condition. Taken together, these data suggest that association with membrane rafts may regulate both AQP2 apical sorting and endocytosis. | | | 19923410
 |
Target gene specificity of USF-1 is directed via p38-mediated phosphorylation-dependent acetylation.
Sébastien Corre,Aline Primot,Yorann Baron,Jacques Le Seyec,Colin Goding,Marie-Dominique Galibert
The Journal of biological chemistry
284
2009
요약 표시
How transcription factors interpret the output from signal transduction pathways to drive distinct programs of gene expression is a key issue that underpins development and disease. The ubiquitously expressed basic-helix-loop-helix leucine zipper upstream stimulating factor-1 binds E-box regulatory elements (CANNTG) to regulate a wide number of gene networks. In particular, USF-1 is a key component of the tanning process. Following UV irradiation, USF-1 is phosphorylated by the p38 stress-activated kinase on threonine 153 and directly up-regulates expression of the POMC, MC1R, TYR, TYRP-1 and DCT genes. However, how phosphorylation on Thr-153 might affect the activity of USF-1 is unclear. Here we show that, in response to DNA damage, oxidative stress and cellular infection USF-1 is acetylated in a phospho-Thr-153-dependent fashion. Phospho-acetylated USF-1 is nuclear and interacts with DNA but displays altered gene regulatory properties. Phospho-acetylated USF-1 is thus proposed to be associated with loss of transcriptional activation properties toward several target genes implicated in pigmentation process and cell cycle regulation. The identification of this critical stress-dependent USF-1 modification gives new insights into understanding USF-1 gene expression modulation associated with cancer development. 기사 전문 | | | 19389701
 |
Distinct targets of the Eco1 acetyltransferase modulate cohesion in S phase and in response to DNA damage.
Heidinger-Pauli, JM; Unal, E; Koshland, D
Molecular cell
34
311-21
2009
요약 표시
Chromosome segregation and the repair of DNA double-strand breaks (DSBs) require cohesin, the protein complex that mediates sister chromatid cohesion. Cohesion requires both a chromatin binding step and a subsequent tethering step called cohesion generation. Here we provide insight into how cohesion generation is restricted to S phase but can be activated in G2/M by a DSB in budding yeast. We show that Wpl1p inhibits cohesion in G2/M. A DSB counteracts Wpl1p and stimulates cohesion generation by first inducing the phosphorylation of the Mcd1p subunit of cohesin. This phosphorylation activates Eco1p-dependent acetylation of Mcd1p, which in turn antagonizes Wpl1p. Previous studies show that Eco1p antagonizes Wpl1p in S phase by acetylating the Smc3p subunit of cohesin. We show that Mcd1p and Smc3p acetylation antagonize Wpl1p only in their proper context. Thus, Eco1p antagonizes Wpl1p in distinct ways to modulate cohesion generation during the cell cycle and after DNA damage. | | | 19450529
 |